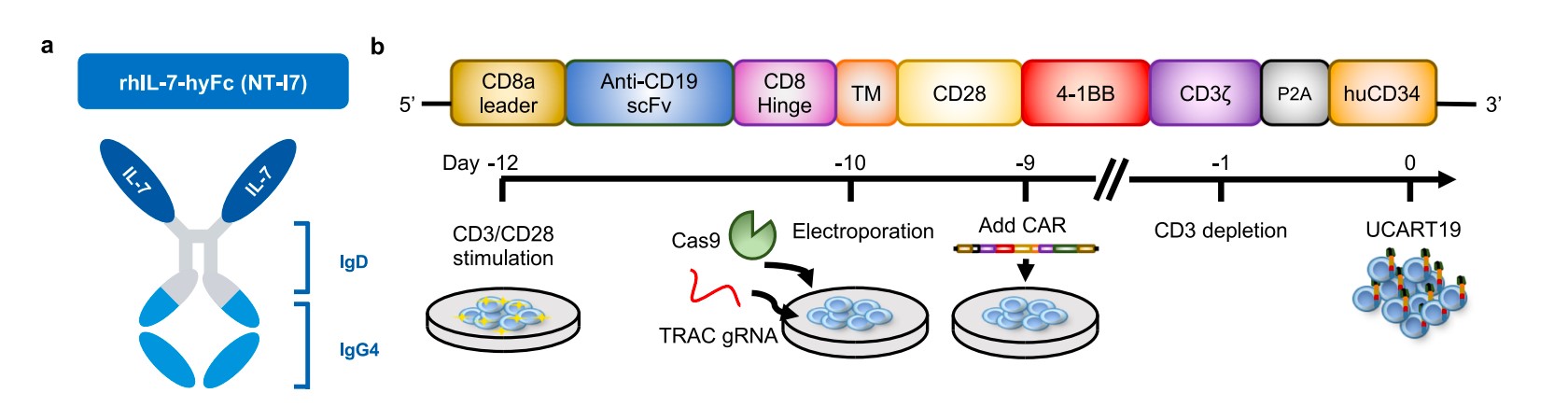
Chimeric antigen receptor (CAR) T cell therapy is a modern adoptive immunotherapy used to treat patients with relapsed/refractory B cell malignancies. However, clinical results showed that CAR-T cells with suboptimal cytotoxicity and reduced persistence could lead to antigen-positive tumor escape and disease relapse. Interleukin-7 is one of the growth factors related to T cell proliferation and survival. rhIL-7-hyFc, a form of interleukin-7 with longer in vivo serum half-life, was demonstrated to improve CAR-T cell expansion, persistence, and anti-tumor efficacy.
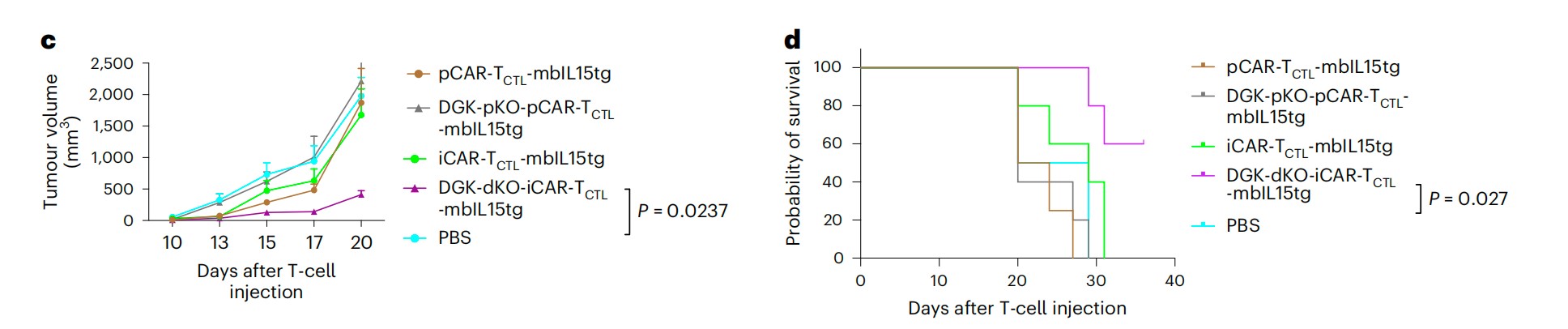
Solid tumors are more refractory to chimeric antigen receptor (CAR) T-cell immunotherapies compared with hematological malignancy. Effector T-cell function was reduced in the tumor microenvironment, including accessibility, expansion, and persistent cytotoxicity.
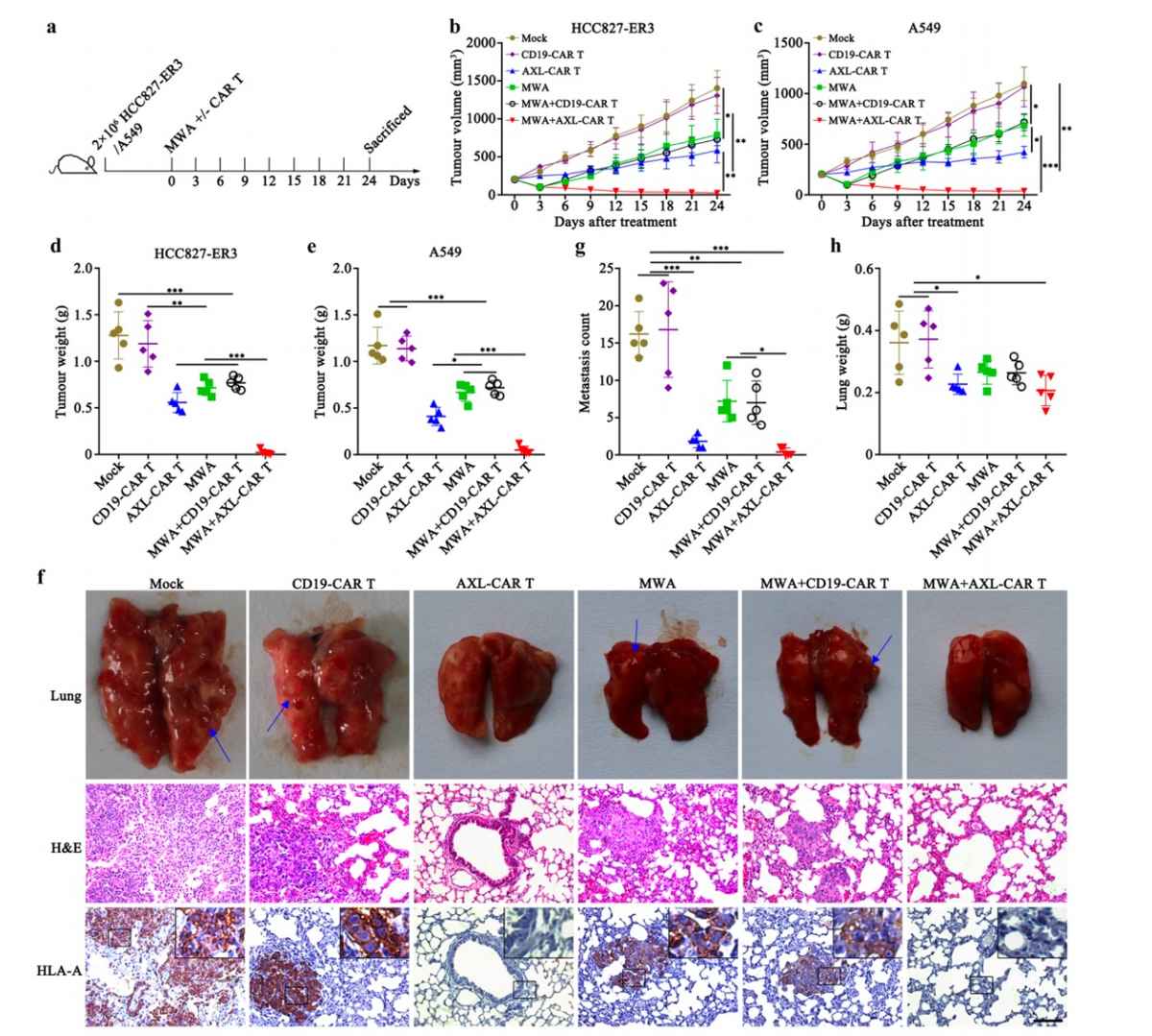
The tumor microenvironment (TME) is a complex tissue that promotes tumor cell proliferation while suppressing T cell efficacy and exhausting them. Breaking the barrier requires regulating the TME and identifying tumor-specific antigens.
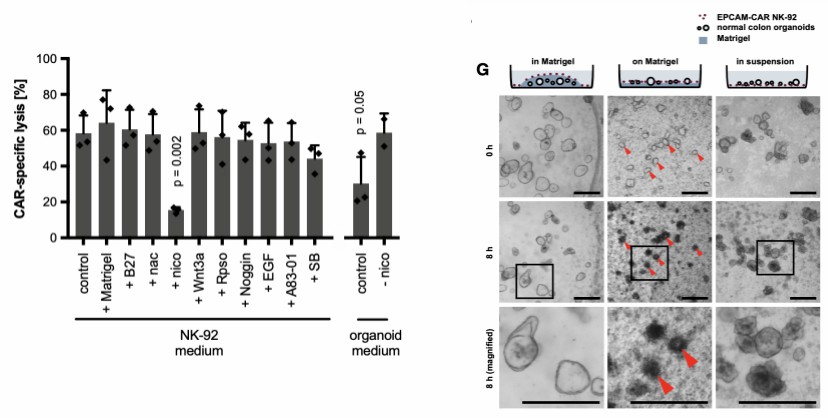
Though CAR-T therapy has achieved success in leukemia treatment, extending the success to solid tumors is challenging due to limited knowledge of the complex solid tumor microenvironment. 3D cancer organoid models provide physiologically associated in vitro tissue models for car-cell therapy anti-tumor efficacy evaluation.
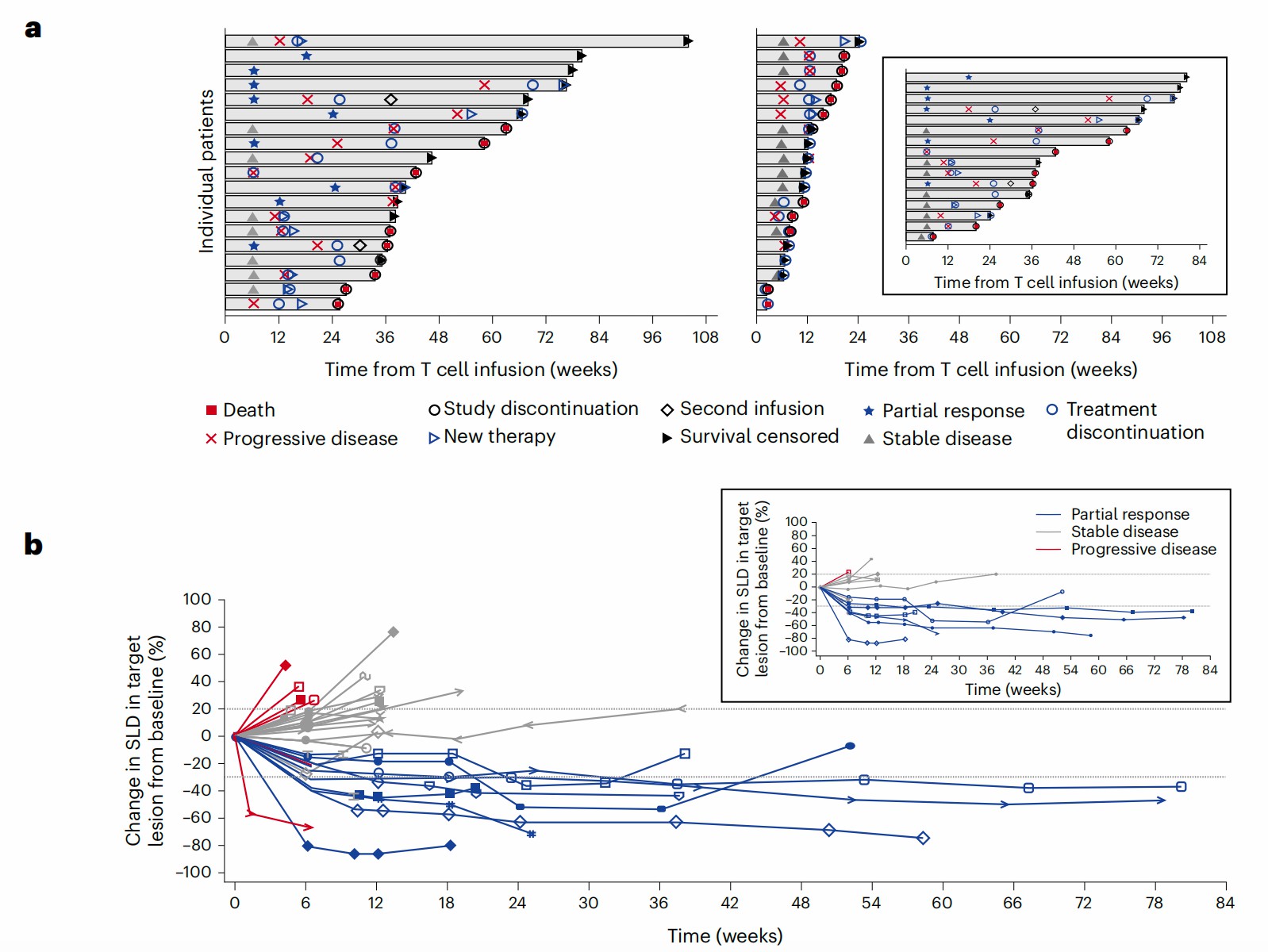
Melanoma-associated antigen A4, also named MAGE-A4, is a potential tumor-associated antigen overexpressed on various solid tumors. The low affinity of natural TCR binding on MAGE-A4 epitope inspired scientists to engineer the T cell with exogenous TCR for antigen recognition and induce enhanced antitumor activities.
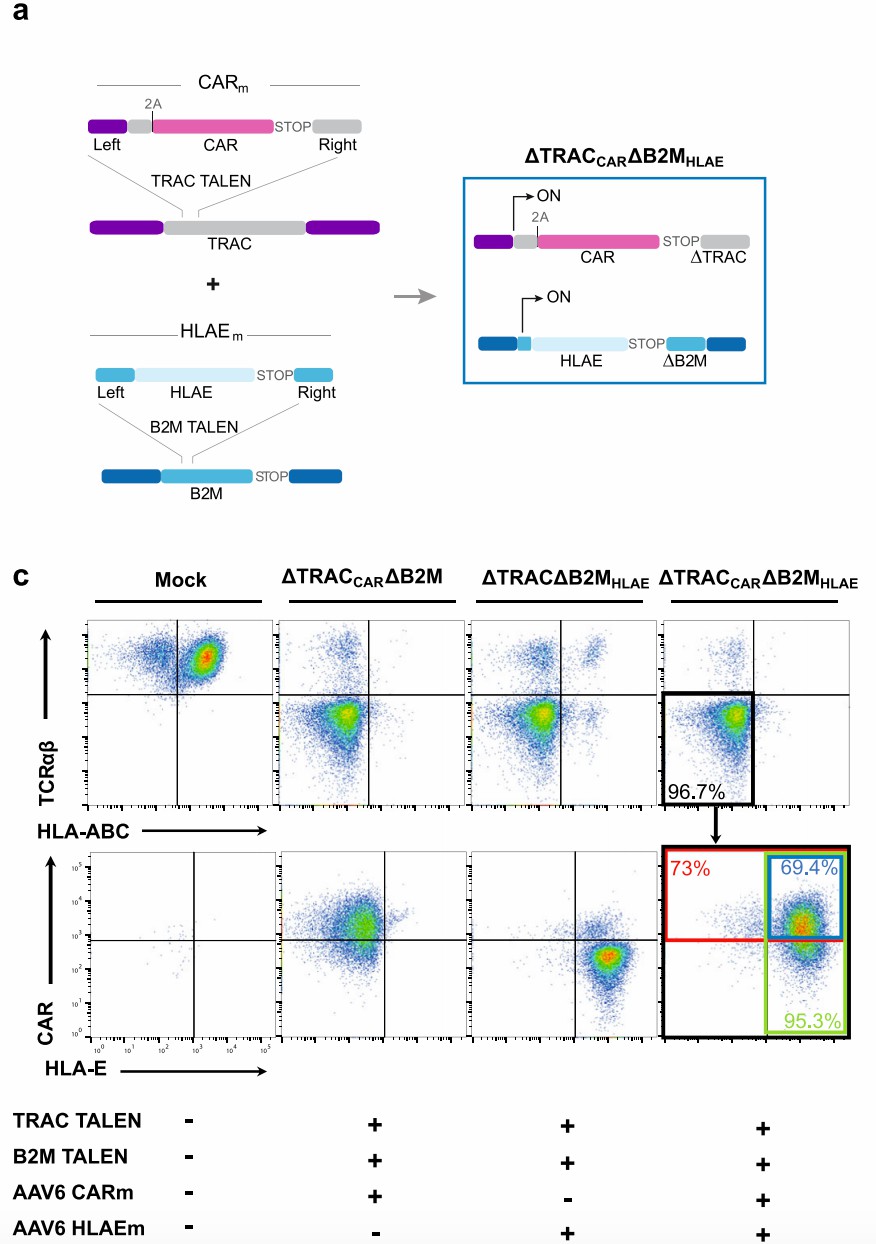
Universal CAR-T is produced from healthy donor T cells and is engineered to kill tumor cells in allogeneic situations. To achieve this aim, TAL Effector Nucleases (TALEN) technology combined with recombinant adeno-associated virus particles (AAV6) are used to engineer T cells.
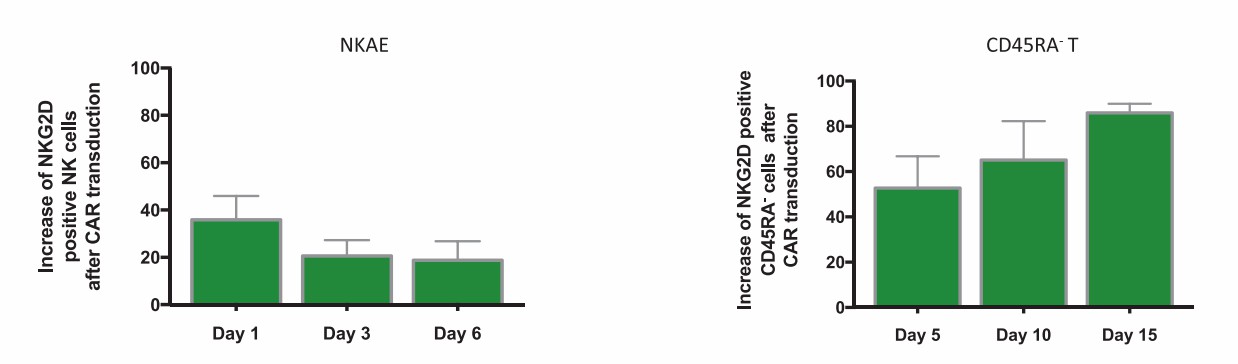
CAR therapy is an attractive strategy for cancer treatment. CAR-NK cells are promising immunotherapy for multiple myeloma patients due to strong cytotoxicity to tumor cells and weak side effects to normal cells. NKG2D ligands show an increased expression in a large proportion of cancers and can be targeted by NKG2D expressed in NK cells and most T cells. In this article, researchers developed autologous NKG2D-CAR-NK cells for MM patients.
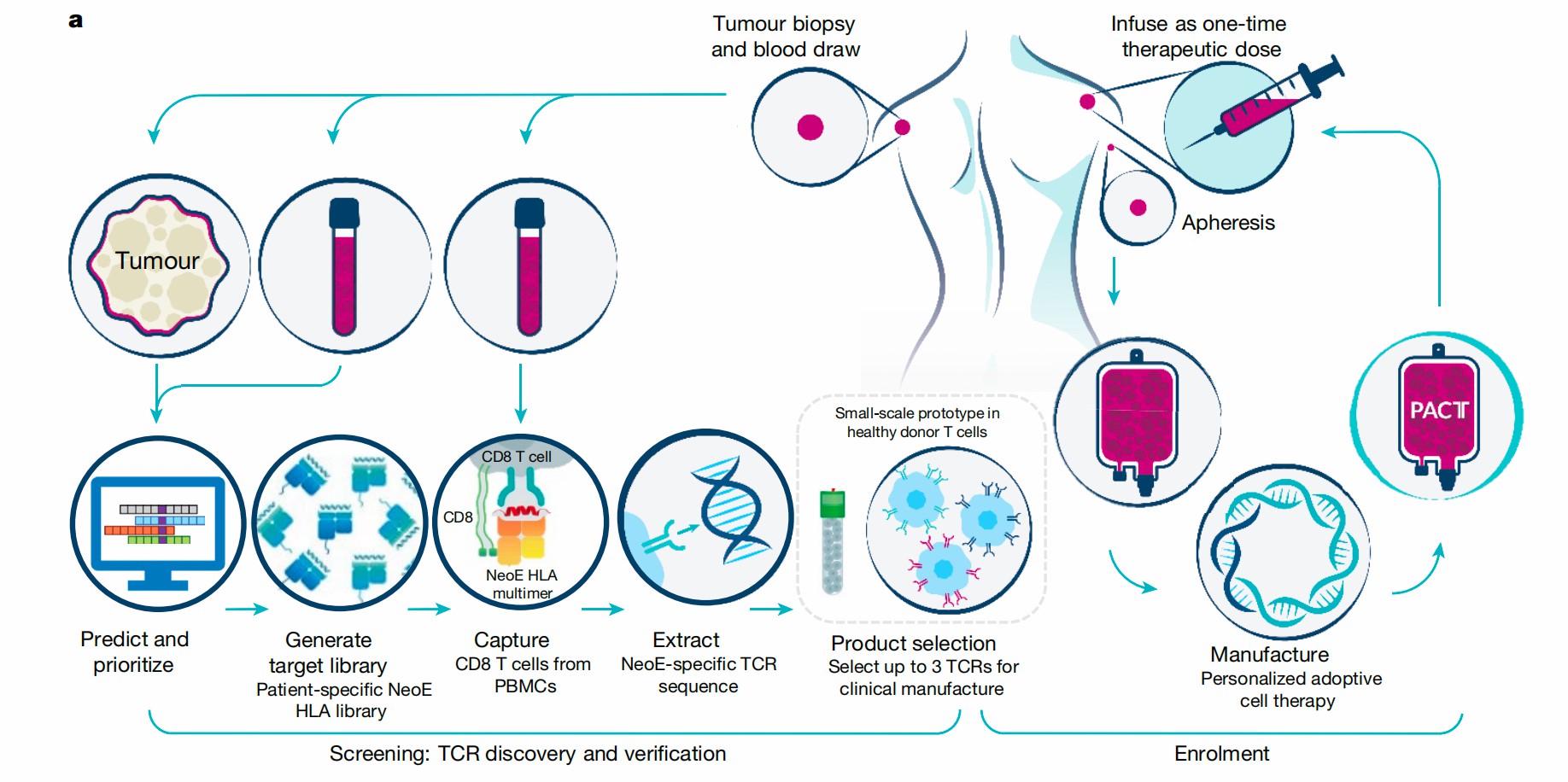
TCR-T is a promising sensitive and specific adoptive cell transfer therapy for solid tumor treatment. In this article, scientists revealed a protocol for personalized T-cell receptor-modified T-cell production. They screened patient tumor-specific antigen peptides and corresponding TCRs using DNA and RNA sequence methods and developed autogenous TCR-modified T cells using non-viral techniques.
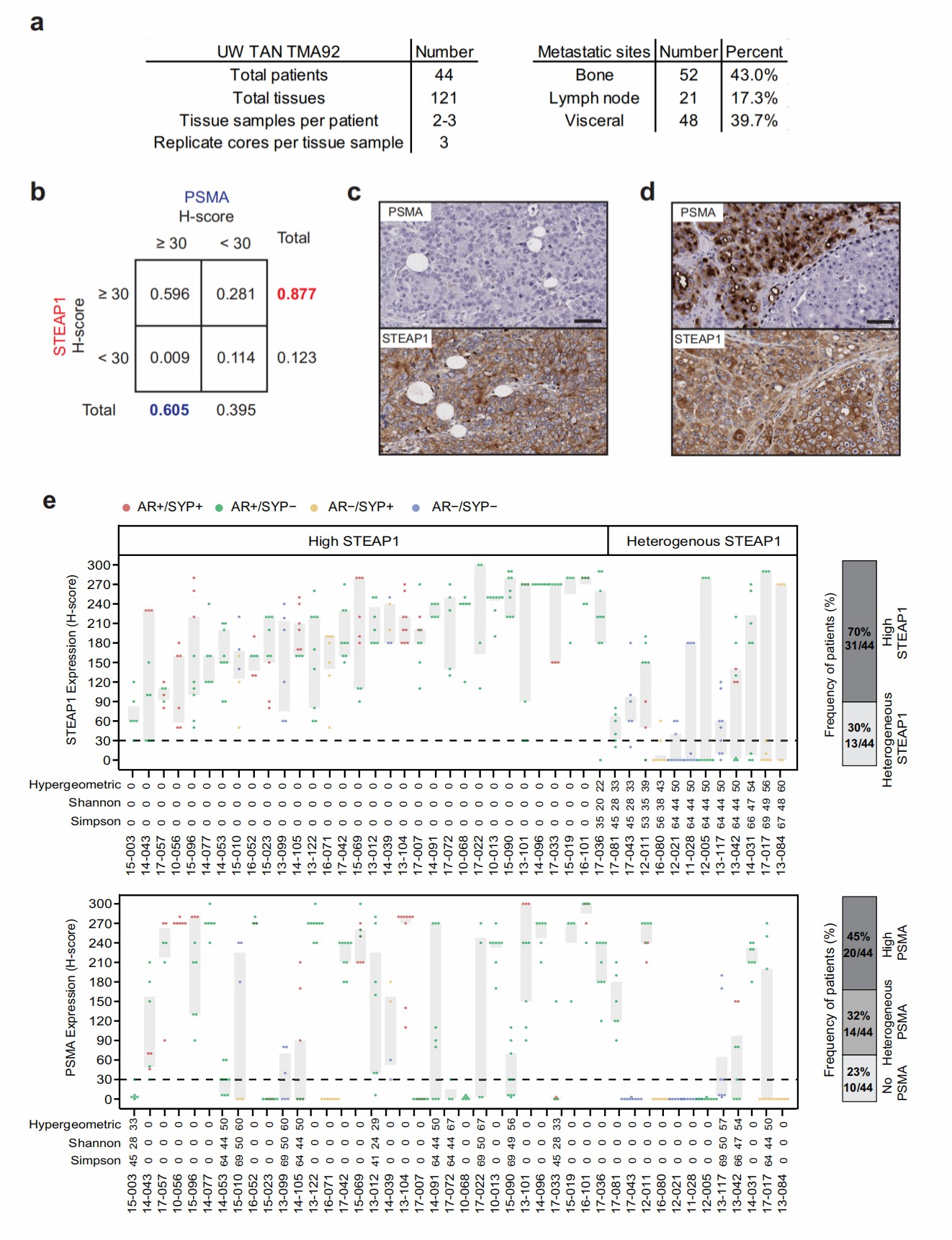
Metastatic castration-resistant prostate cancer (mCRPC) is the late stage of prostate cancer. Researchers found that STEAP1, a six transmembrane epithelial antigen of the prostate 1, is highly expressed on prostate tumor cells and presents a more frequent and homogeneous expression in advanced prostate tumors.
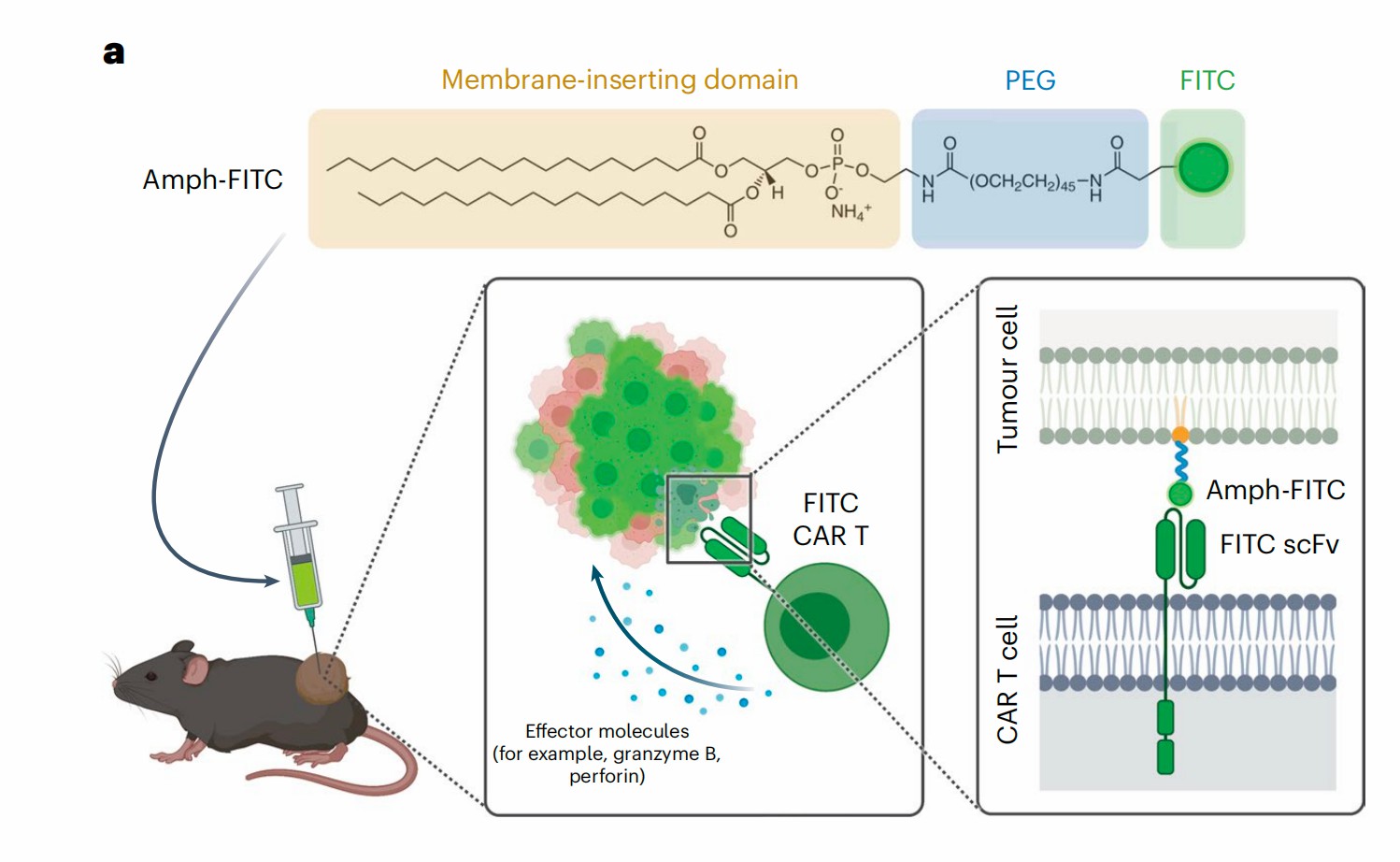
Good clinical results of chimeric antigen receptor (CAR) engineered T cell therapy in hematological malignancies has brought great expectation in translating the success to solid tumor eradication. However, the application of CAR-T in solid tumor treatment is hindered by the lack of proper antigen targets and the complex tumor microenvironment.
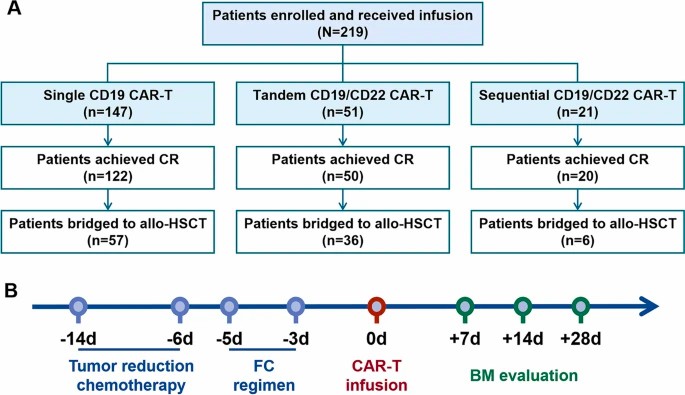
CAR-T cells targeted to CD19 antigen on B cells have achieved great clinical results in B-cell acute lymphoblastic leukemia (B-ALL) treatment. Dual-target CAR-T cells were developed to decrease the possibility of relapse induced by antigen escape after receiving CD19 CAR-T therapy.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION