Creative Biolabs specializes in T-cell receptor (TCR) development and analysis services. We also provide a cytokine release assay service designed to evaluate the functionality of TCR-modified T cells. This service offers a detailed analysis of the cytokine release capability, enabling us to detect and quantify the quantity and specific types of cytokines secreted by these engineered T cells, assisting our customers in understanding their performance.
Creative Biolabs offers a range of measurement methods, including enzyme-linked immunospot assay (ELISpot), intracellular cytokine staining assay (ICS), and flow cytometry, among others. These techniques are used to assess the performance of modified T cells in response to viral or tumor antigens. The modified T cells are capable of producing elevated levels of cytokines like IFN-γ, IL-12, and GM-CSF. Creative Biolabs focuses on these specific cytokines and employs suitable methods to analyze their release. Our platforms are tailored to meet the specific requirements of the therapy being assessed and can be customized as per client needs.
1. ELISA / ELISpot (Enzyme-Linked Immunospot Assay)
ELISA is a widely used and highly sensitive technique for quantifying cytokines in a sample. It allows for the simultaneous measurement of multiple cytokines and is well-suited for both in vitro and in vivo applications. ELISpot is used to measure and quantify the release of cytokines from individual cells within a sample.
2. Flow Cytometry
Flow cytometry enables the identification and quantification of cytokine-secreting cells within a heterogeneous population. This platform provides valuable insights into the cellular source of cytokine production.
3. Multiplex Bead-Based Assays
Creative Biolabs offers multiplex assays that can simultaneously analyze a panel of cytokines. This approach is particularly beneficial when a comprehensive assessment of the cytokine profile is needed.
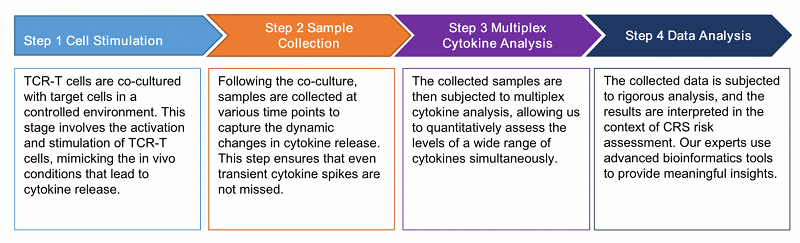
The workflow of the Cytokine Release Assay is a meticulously designed process aimed at providing accurate and reliable results. The workflow encompasses the following essential steps:
Creative Biolabs offers a solution to help you reclaim your valuable time for essential core work and projects. If you have any particular requirements or would like to explore how we can assist you, please don't hesitate to get in touch with us to discuss your demands or request a proposal.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION