The specificity of the antibody is very important. One of the most common and effective methods to test the specificity of antibodies is the antigen blocking test. Creative Biolabs provides human leukocyte antigen (HLA) blocking assay to test the specificity of HLA antibodies.
A combination of blocking antigen and antibody is used to block the binding of antibodies and specific antigens in the process of antibody and natural antigens binding. The binding of antibodies and antigens is well specific if the binding of antibodies and natural antigens can be blocked by the characteristics of the antigen. Antigen blocking test is an effective method for all the antibodies, including the specificity of the Immunofluorescence (IF)/immunocytochemistry (ICC) antibody.
The process of antigen blocking experiments for IF/ICC antibody refers to incubation of antigen and antibody firstly, and then the mixture was added to the natural cells or tissues, which make antibodies and natural antigen binding. The blocking antigen includes proteins or polypeptides, which have been used to prepare the IF/ICC antibody.
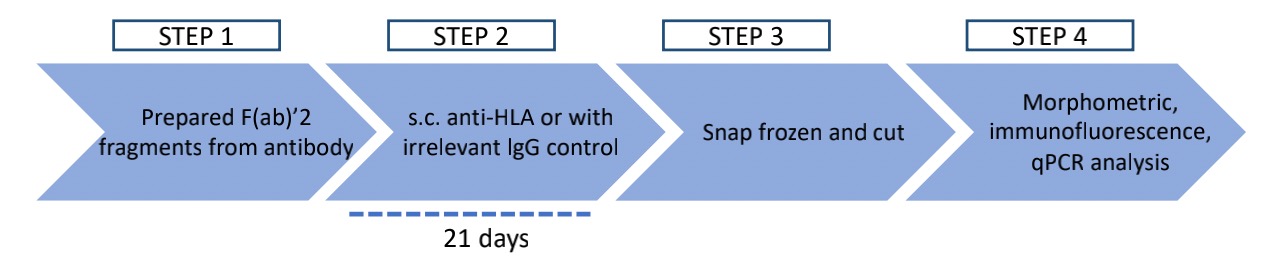 Fig.1 Workflow of in vivo HLA blockade.
Fig.1 Workflow of in vivo HLA blockade.
MHC class II blockade reduces allogeneic T cell-mediated injury and CD8+ CTL-associated transcripts in human arterial grafts. Existential studies have proven this view by in vivo HLA-DR blocking assay. Here are some relevant findings as follows:
Anti-HLA-DRα F(ab)'2 fragment significantly reduces total intimal T cell infiltration as detected by IF (A) and CD8+ T cell-associated transcripts of cytokines and CTL effector molecules in rejected artery grafts at 21 days (B) as detected by qPCR normalized to either total mRNA or T cell-specific mRNA.
In an MHC-mismatched model of arterial rejection by allogeneic T cells, blockade of class II MHC by anti-HLA-DRα F(ab)'2 fragment reduces intimal area expansion and increases lumen area as measured by H&E and EVG staining (A) and reduces disruption of enterochromaffin cell (EC) lining the vessel lumen, a hallmark of intimal arteritis/endothelialitis, as measured by percent of circumferential coverage (B).
With the in-depth study of HLA, Creative Biolabs offers a complete range portfolio of assays for HLA Testing, including HLA blocking assay. If you are interested in our HLA blocking assay, please feel free to contact us directly.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION