Though CAR-T cell therapy is efficient in cancer treatment, the production process is time-consuming. The classical CAR-T production steps involve T cell isolation from peripheral blood, lentivirus packaging, CAR gene delivery, and in vitro large-scale expansion, which typically needs two weeks for manufacturing. A rapid CAR-T production strategy is urgently needed to reduce the waiting time before graft and the loss of potency during long-term cultivation and differentiation. Creative Biolabs is a well-recognized biotech providing advanced CAR-T products and services for global clients, and now we present rapid CAR-T production service for global clients. The rapid CAR-T cells produced using modified approaches retain stem cell-like features and perform durable antitumor functions.
With Creative Biolabs' innovative rapid CAR-T production platform, CAR-T cells are rapidly produced from non-activated, primary T cells within a single day with optimized cell culture conditions and high-titter lentiviral vectors.
Isolate mononuclear cell or peripheral blood T cells.
Specialized non-activated primary T cells culture medium.
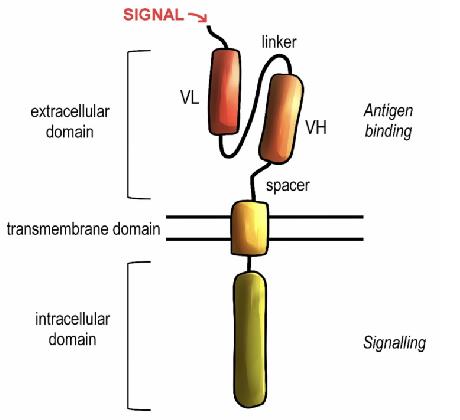
CAR lentiviral packaging vector design and construction.
Introducing necessary cytokine fusion proteins to lentiviral vectors.
High-quality pseudovirus particles production.
Special cell culture medium for lentiviral transduction.
Optimized cell culture conditions.
Highly efficient lentivirus diffusion and transduction.
Collect CAR-T cells after lentivirus transduction for 24 hours
Test CAR expression using an immunostaining assay
Flow cytometry-based cell phenotyping.
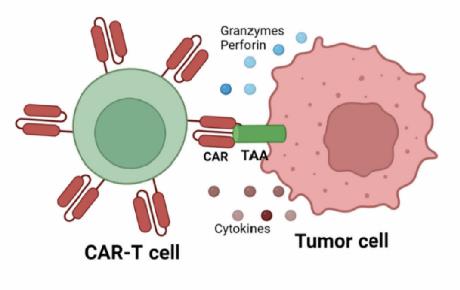
Animal models based in vivo efficacy tests
CAR-T in vivo growth and persistence.
| Items | Long-term Culturing CAR-T | Rapid CAR-T |
| Original Cell | Primary T cell | Primary T cell |
| Activation | CD3/CD28 Antibodies | None |
| Lentivirus Transduction Time Points | At dividing stage, after activation for 1-3 days. | At non-dividing stage, direct transduction after isolation. |
| Lentivirus Transduction Efficiency | High | Low, can be enhanced by various strategies |
| Expansion Period | 6-9 Days | 1 Day |
| Cell Property | Effector memory T cell | Stem cell-like, memory T cell |
| Antitumor Efficacy After Engraftment | Variant responses, regression, or relapse. | Control tumor burden, persist for long-period. |
Creative Biolabs has developed a reliable naive T cell transduction strategy for rapid CAR-T cell production. If you have any problem with CAR-T development, please contact us and discuss your program with our experts.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION