All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs is proud to offer a wide range of CD79B CART-related products, such as CAR viral particles and CAR cells.
CD79b plays an important role in the signaling complex of B cell receptors (BCRs) and is crucial for the development and maintenance of mature B cells. Encouragingly, preliminary evidence suggests that targeting CD79b using antibody-drug conjugates, or CD79b CART therapies exhibits promising efficacy.
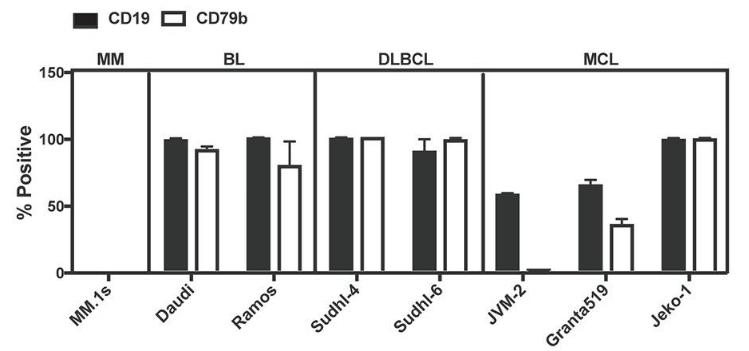 Fig.1 The expression of CD79b and CD19 on human B cell tumor cell lines.1
Fig.1 The expression of CD79b and CD19 on human B cell tumor cell lines.1
We offer a full range of CD79B protein and cell products, which have been widely used for the expression test of anti-CD79B CAR-T cells.
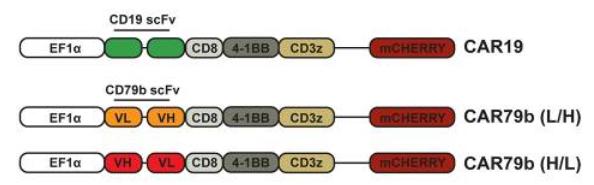 Fig.2 Structure of two second-generation chimeric antigen receptors.1
Fig.2 Structure of two second-generation chimeric antigen receptors.1
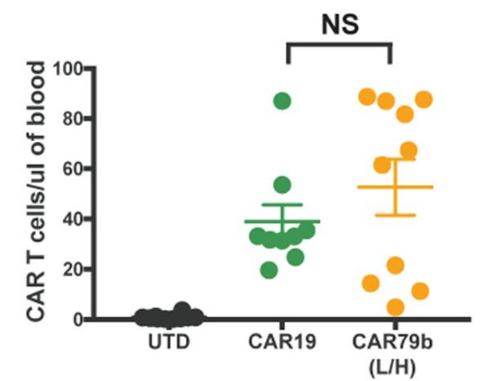 Fig.3 Absolute numbers of CAR T cells in blood.1
Fig.3 Absolute numbers of CAR T cells in blood.1
CD79B CAR-T Cytokine Release Test
The CD79B CAR-T cytokine release test service aims to assess the potential for cytokine release and evaluate the immune response upon administration of CD79B CAR-T cells. This service provides comprehensive analysis and monitoring of various cytokines released, including IL-6, TNF-α, IFN-γ, and IL-10.
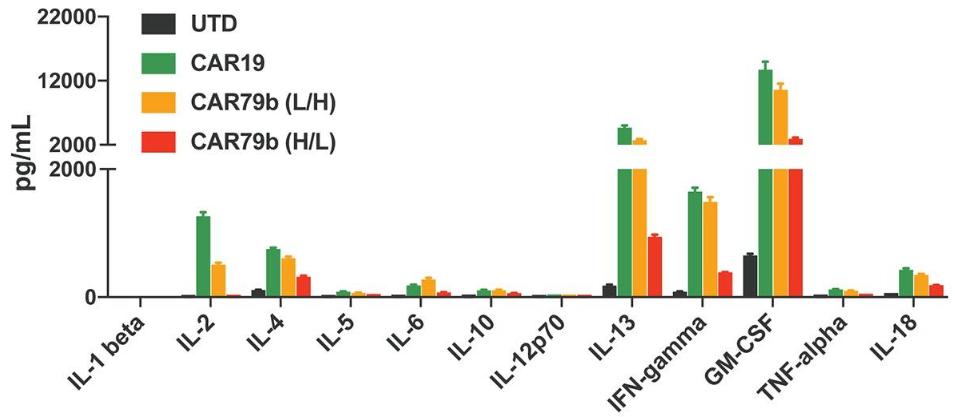 Fig.4 Effector cytokine production after co-culture of CAR T cells.1
Fig.4 Effector cytokine production after co-culture of CAR T cells.1
CD79B CAR-T In Vitro Cytotoxicity Assay
This powerful assay allows for the evaluation of the cytotoxic activity of these engineered T cells against CD79B-expressing cancer cells. The assay measures the ability of the CAR-T cells to effectively recognize and kill the target cells.
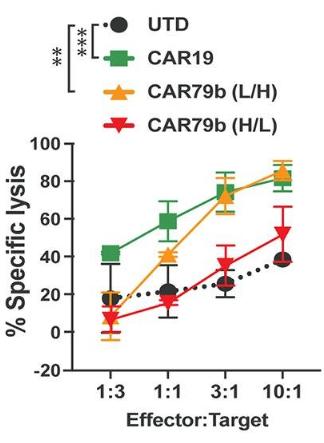 Fig.5 Cytotoxicity of CD79B CAR-T cells.1
Fig.5 Cytotoxicity of CD79B CAR-T cells.1
CD79B CAR-T Cell Proliferation Test
Our CD79B CAR-T cell proliferation test service offers a comprehensive analysis of the proliferation capacity of CD79B CAR-T cells. This service aims to evaluate the expansion and longevity of CD79B CAR-T cells in vitro, providing valuable insights into their potential efficacy and durability.
CD79B CAR-T Cell Therapy Animal Models
Animal models are designed to closely mimic the human immune system and tumor microenvironment, providing valuable insights into the potential therapeutic outcomes and toxicities of the treatment. Various animal models, including mice, rats, and primates, are used to study different aspects of CD79B CAR-T cell therapy.
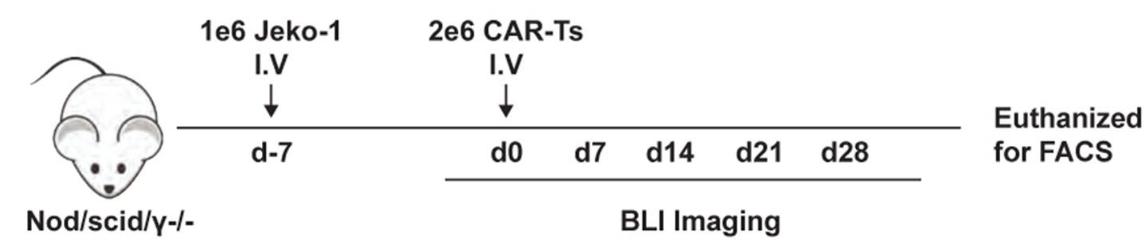 Fig.6 Schematic overview of xenograft experiment.1
Fig.6 Schematic overview of xenograft experiment.1
Our CD79B CAR-T efficacy test is to assess the response rate, duration of response, and overall survival in mouse models. The efficacy test is an essential step in evaluating the effectiveness and safety of this therapeutic approach.
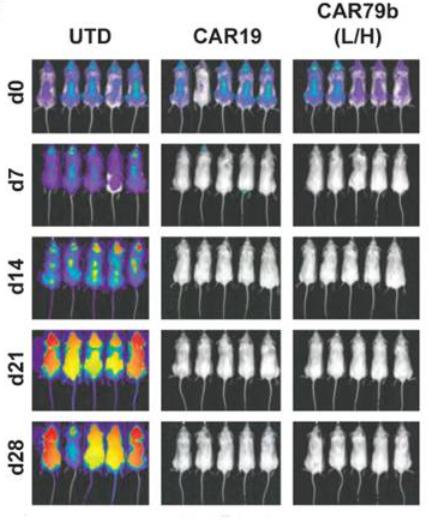 Fig.7 Analysis of tumor burden over time for one experiment.1
Fig.7 Analysis of tumor burden over time for one experiment.1
Toxicity Evaluation of CD79B CAR-T
With our extensive knowledge in in vivo research, project management, and utilization of appropriate models, Creative Biolabs presents CAR-T cell toxicity assessment service. We specialize in designing comprehensive research protocols and conducting dependable high-standard in vivo studies.
Reference
 Loading...
Loading...
| CAT | Product Name | Target Species | Antibody Clone | Antibody Host | Receptor Construction | Vector Type | Targeting Cell Type | CAR Vector Type | Inquiry & Datasheet |
| XS-1122-LX202 | Anti-CD79B (X11X202) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X202 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX203 | Anti-CD79B (X11X203) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X203 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX204 | Anti-CD79B (X11X204) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X204 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX205 | Anti-CD79B (X11X205) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X205 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX206 | Anti-CD79B (X11X206) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X206 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX207 | Anti-CD79B (X11X207) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X207 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX208 | Anti-CD79B (X11X208) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X208 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX209 | Anti-CD79B (X11X209) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X209 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX210 | Anti-CD79B (X11X210) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X210 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX211 | Anti-CD79B (X11X211) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X211 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX212 | Anti-CD79B (X11X212) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X212 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX213 | Anti-CD79B (X11X213) h(CD28-41BB-CD3ζ) CAR, pCDCAR1 | Human | X11X213 | Humanized | scFv-CD28-41BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX590 | Anti-CD79B (X11X192) h(CD28-OX40-CD3ζ) CAR, pCDCAR1 | Human | X11X192 | Humanized | scFv-CD28-OX40-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX609 | Anti-CD79B (X11X211) h(CD28-OX40-CD3ζ) CAR, pCDCAR1 | Human | X11X211 | Humanized | scFv-CD28-OX40-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX610 | Anti-CD79B (X11X212) h(CD28-OX40-CD3ζ) CAR, pCDCAR1 | Human | X11X212 | Humanized | scFv-CD28-OX40-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX988 | Anti-CD79B (X11X192) h(ICOS-4-1BB-CD3ζ) CAR, pCDCAR1 | Human | X11X192 | Humanized | scFv-ICOS-4-1BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX989 | Anti-CD79B (X11X193) h(ICOS-4-1BB-CD3ζ) CAR, pCDCAR1 | Human | X11X193 | Humanized | scFv-ICOS-4-1BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX990 | Anti-CD79B (X11X194) h(ICOS-4-1BB-CD3ζ) CAR, pCDCAR1 | Human | X11X194 | Humanized | scFv-ICOS-4-1BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX991 | Anti-CD79B (X11X195) h(ICOS-4-1BB-CD3ζ) CAR, pCDCAR1 | Human | X11X195 | Humanized | scFv-ICOS-4-1BB-CD3ζ | Lentiviral vector | T cell | ||
| XS-1122-LX992 | Anti-CD79B (X11X196) h(ICOS-4-1BB-CD3ζ) CAR, pCDCAR1 | Human | X11X196 | Humanized | scFv-ICOS-4-1BB-CD3ζ | Lentiviral vector | T cell |
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION