The extracellular matrix (ECM) regulates the development and maintains tissue homeostasis. The ECM is comprised of approximately 300 proteins, and they serve a variety of functions. Some cross-link to form into long filaments that in turn bundle into fibers and serve largely a structural role. In addition to its structural role, the ECM takes part in most basic cell behaviors, from cell proliferation, adhesion and migration, to cell differentiation and cell death. ECM is dynamically remodeled and specifically tailored to the structure/function of each organ, and its composition, biomechanics and anisotropy are exquisitely tuned to reflect the physiological state of the tissue. However, the dysregulation of ECM is a remarkable feature of the tumor microenvironment (TME). During the development of cancer, malignant cells contribute to ECM stiffness, and, in return, the stiffened ECM alters the characteristics of cancer cells. The increased production and density of ECM confers the TME with tumor-promoting and cell-spreading properties.
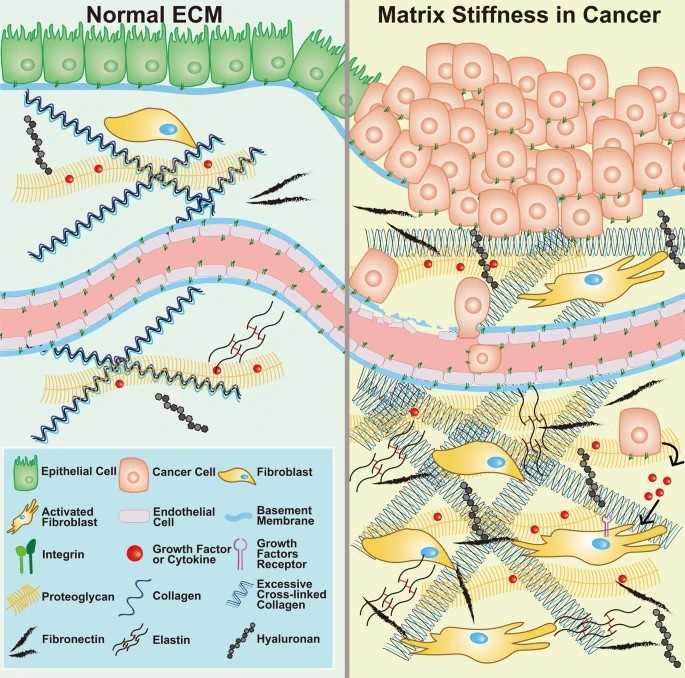 Fig.1 Schematic illustration of ECM components in normal tissue (left) and the TME (right).1,3
Fig.1 Schematic illustration of ECM components in normal tissue (left) and the TME (right).1,3
Alteration in the density and composition of ECM occurs in tumors. Many solid tumors express high levels of various ECM molecules like fibrillar collagens, fibronectin, elastin, and laminins. The Source of these ECM molecules are the tumor cells themselves but to an even larger degree cancer-associated fibroblasts (CAFs). The cancer-associated ECM is not only an integral feature of a tumor but also actively contributes to its histopathology and behavior.
ECM is a major component of TME and plays a critical role in cancer development and progression. During tumor progression, tumor cells commonly modify and hijack the surrounding ECM to sustain anchorage-dependent growth and survival, guide migration, store pro-tumorigenic cell-derived molecules and present them to enhance receptor activation. Moreover, EMC can modulate the immune microenvironment. ECM proteins can recruit immunosuppressive cells while simultaneously blocking the recruitment of anti-tumorigenic immune cells. In addition, the ECM composition can dramatically modulate the activation state of the tumor-infiltrating immune cells.
The ECM mediates cancer development through several mechanisms, including the formation of a physical barrier to anti-cancer drugs, provision of growth factor and cytokines reserves, alteration of immune cell responses, stimulation of integrin-dependent signaling that promotes invasion and proliferation, and establishment of an advantageous niche for metastatic cells.
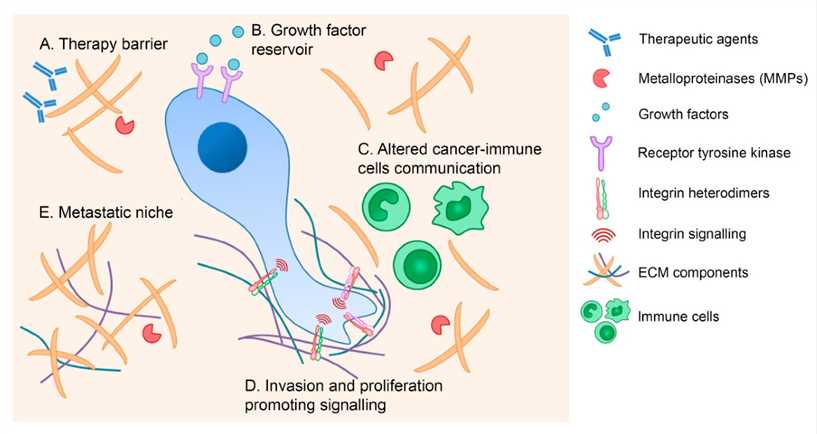 Fig.2 The ECM contribution to cancer pathogenesis.2,3
Fig.2 The ECM contribution to cancer pathogenesis.2,3
Targeting the components of ECM is a therapeutic approach to remodel TME. The modulation of ECM-related enzymes such as collagenase, matrix metalloproteinases (MMPs) or lysyl oxidases can be a promising therapeutic strategy. Because β1-integrins interact with multiple ECM components including collagen I, β1-integrin antagonists may have unintended effects. In fact, the success of integrin-targeted strategies in cancer has been limited. In addition, the pharmaceutical industry has worked to develop safe therapies to inhibit MMP activity. Although existing literature supports targeting ECM components as a promising therapeutic strategy, near depletion of the stroma may compromise or even worsen the outcomes.
Creative Biolabs offers the most comprehensive services for reshaping TME. Please feel free to contact us for more information.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION