The advent of Chimeric Antigen Receptor T-cell (CAR-T) therapies has revolutionized the field of oncology, providing new avenues for treating various forms of cancer. Our 20 years of experience in the biotechnology sector have equipped us with the expertise and technological capabilities to support the entire lifecycle of CAR-T products, from early development to commercialization.
The biopharmaceutical industry has witnessed a significant evolution in recent years, characterized by the increasing complexity of therapeutic molecules and accelerated timelines. The demand for CAR-T cell therapies has surged due to their potential to provide targeted and personalized treatment for various cancers. Thus, CDMO companies face the dual challenge of ensuring the highest quality standards while meeting accelerated timelines.
Historically, CDMO projects focused primarily on simpler molecules. However, in recent years, there has been a marked shift towards more complex molecule formats, including chimeric antigen receptor (CAR) T cells. This complexity requires a higher level of technical competence.
Maintaining the highest quality standards is non-negotiable. Strict adherence to Good Manufacturing Practices (GMP) for the development and supply of materials for toxicology studies is critical. Quality assurance must permeate each stage of the development and manufacturing process, from the selection of raw materials to the testing of the final product.
The biopharmaceutical industry is driven by intense market competition, regulatory requirements, and the need to maintain patent exclusivity. As a result, customers expect not only shorter, but also reliable schedules. To minimize development costs, meet patient needs, and meet investor expectations, an accelerated timeline is critical.
Creative Biolabs acts as your fully integrated strategic partner, addressing the core technical and logistical challenges inherent in advanced cell therapy development. We offer a unified solution that eliminates the risk, cost, and time of coordinating multiple specialized vendors for critical raw material and drug product manufacturing.
Specific deliverables and solutions you can expect:
Discover How We Can Help - Request a Consultation.
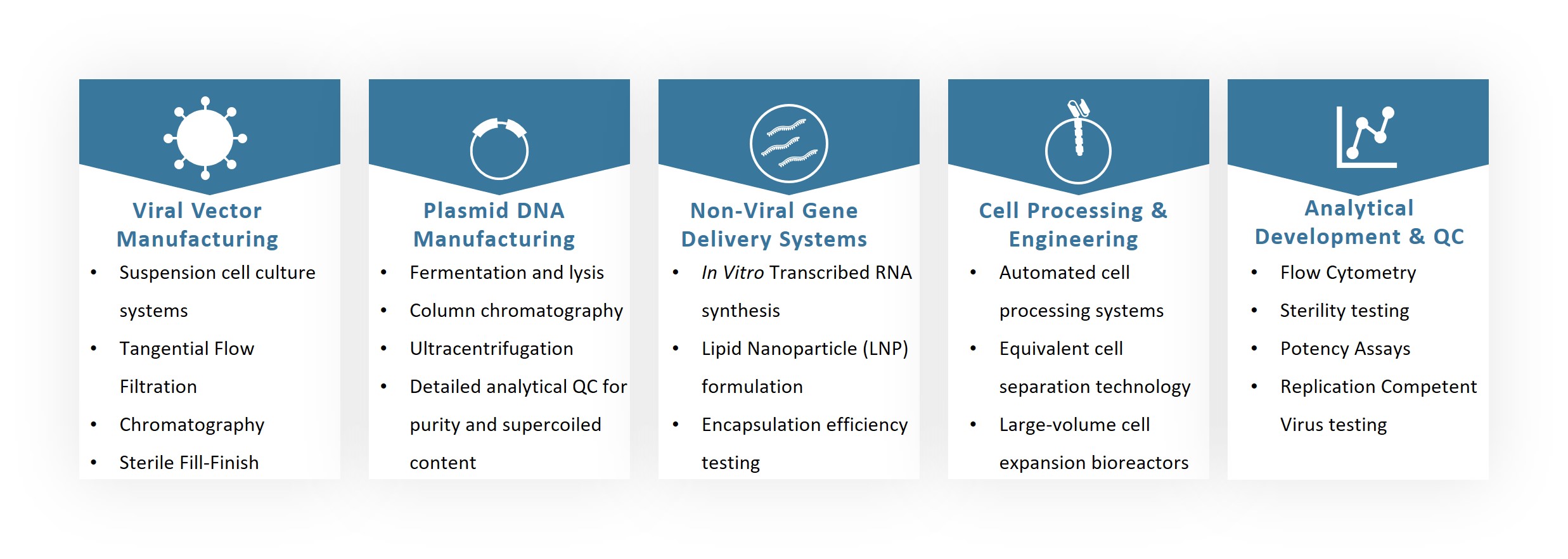
Creative Biolabs offers a comprehensive set of established, integrated technology platforms designed to manage the entire lifecycle of a cell therapy product, from early development through to cGMP manufacturing.
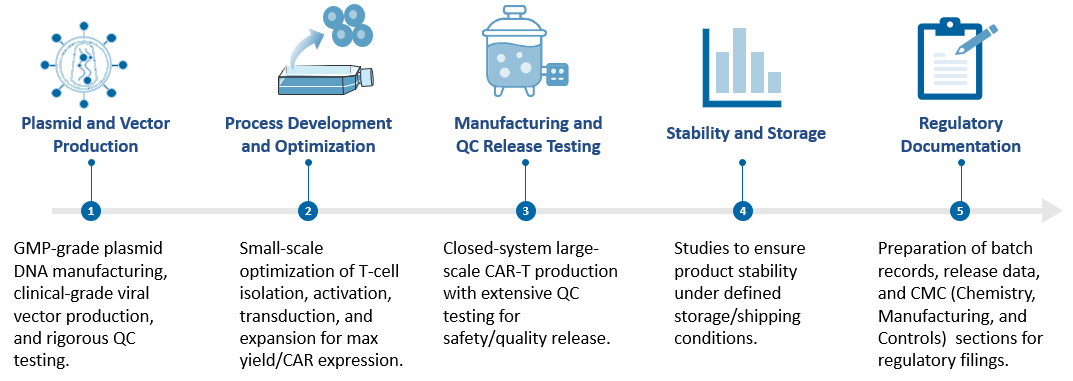
The development of clinical-grade CAR-T therapy follows a highly structured and quality-driven process. Our workflow ensures a seamless transition across key technical milestones, with a focus on delivering a regulatory-compliant product.
We welcome you to get in touch to dive into the details of your requirements with our team.
Our expertise in process development ensures that CAR-T therapies are scalable and compliant with regulatory requirements. We utilize cutting-edge technologies to optimize cell culture conditions and purification methods, focusing on reproducibility and scalability.
Our in-house analytical capabilities enable rigorous assay development and quality control, ensuring that all CAR-T products meet the highest standards of safety, potency, and purity.
Global CDMO Services for Plasmid
We offer a range of plasmid services, from research grade to GMP-grade plasmid DNA. These services are designed to support various stages of CAR-T development, offering flexibility and reliability.
Global CDMO Services for CAR Viral Vector
Viral vectors are crucial for CAR-T cell engineering. We offer research-grade, GMP-like, and GMP-grade viral vector production, including CGMP AAV, lentivirus, and retrovirus production. Our viral vector services ensure high purity, potency, and safety.
IVT RNA and LNP Manufacturing for CAR Cells
Our mRNA services range from research grade to GMP grade, including lipid nanoparticle encapsulation. This service is crucial for CAR-T cell modification and engineering.
CAR Viral Vector Packaging Services
Creative Biolabs provides comprehensive viral packing services for CART projects, including the production of research-grade viruses, GMP-like viruses, and cGMP-grade viruses such as AAV, lentivirus, and retrovirus.
Global CDMO Services for CAR Cells
We offer various grades of CAR cell production, including IIT grade, IND grade, ensuring that each stage of development is adequately supported.
Collaboration and Transparency
Our approach is highly collaborative and transparent. Clients are invited on-site to work alongside our team, fostering a deeper understanding of the project and enhancing the overall development process.
Quality by Design
Our commitment to quality is ingrained in our ‘Quality by Design’ approach. Process optimization, design of experiments, and rigorous quality control measures ensure that every CAR-T product meets benchmark standards.
Global Reach
With facilities across the globe, Creative Biolabs offers unparalleled flexibility and accessibility. Our services are able to support customers in different regions and provide consistent and high quality CDMO services worldwide.
How does Creative Biolabs ensure the quality of CAR-T products?
We employ a "Quality by Design" approach, focusing on process optimization, rigorous quality control, and strict adherence to GMP standards. Our internal analysis further ensures the safety, efficacy, and purity of the highest standard.
How do you handle the complexity of CAR-T projects?
Our extensive experience and technological capabilities enable us to manage the complexity of CAR-T projects effectively. We offer comprehensive services, from process and analytical development to clinical and commercial manufacturing.
Creative Biolabs is committed to advancing the development and manufacturing of CAR-T cell therapies. Our comprehensive CDMO services, state-of-the-art technology and unwavering commitment to quality ensure that our customers can bring innovative therapies to market efficiently and reliably.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION