Creative Biolabs is dedicated to the advancement of innovative biologic medications in the field of cancer immunotherapy and offers CDMO services for various novel CAB-Mo cell therapy projects, including plasmid, lentivirus, and cell process production. We will collaborate with our clients to propel the innovative CAB-Mo cell therapy program to the next milestone at the earliest opportunity and usher in a new era in cell and gene therapy!
Over the past few decades, CARs have proven their promising role in treating several malignancies, such as B-cell-derived tumors. After the success of CAR-T, researchers have applied this strategy to other immune cells, such as NK cells and Treg.
Monocytes are a type of white blood cell in the human immune system and are usually produced in the bone marrow. Monocytes can participate in immune response and have very strong anti-tumor and anti-viral effects. Therefore, monocytes have become the latest option for the treatment of solid tumors.
In Creative Biolabs, we are interested in developing CAR-mononuclear cells for tumor immunotherapy. The advent of CAR-Mo opens up new possibilities for the treatment of solid tumors: modifying human monocytes with specific CARs to improve the phagocytic activity and antigen presentation of monocytes to tumors.
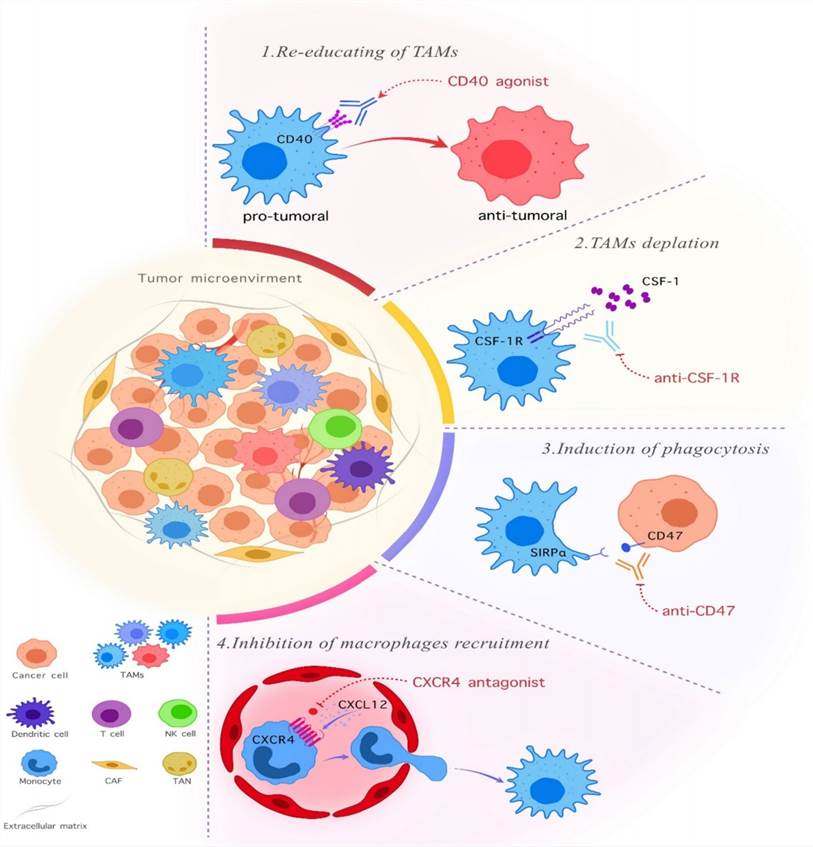 Fig.1 TAM-Targeting Anti-Cancer Response Solutions.1
Fig.1 TAM-Targeting Anti-Cancer Response Solutions.1
In our CAR-Mo platform, we have generated a novel class of human peripheral blood CD14+ CAR-monocytes by using chimeric lentiviral vector CBLpCDH-Lenti™ to stably express anti-CD19 CAR. Normally, an anti-CD19 CAR consists of a scFv specific for CD19, a CD3ζ signaling domain, and a CD28 costimulation domain. Human PBMC suspensions are placed in culture bottles and cultured for 12-20 days. The proliferated and activated mononuclear cells are collected and resuspended in normal saline to a final concentration of 0.5-1×107/ml. Monocytes are finally further purified by positive immunomagnetic selection directed against CD14. Human CD14+ monocytes are then genetically modified with anti-CD19 CAR to show potential immunomodulatory and antitumor activity. Our data have indicated that CAR monocytes had direct antitumor activity. The cell viability and positive rate of CAR cells are both over 85%.
Drawing on extensive research experience in tumor immunity, Creative Biolabs offers comprehensive support for CAR-Mo and other cell therapy research and development, including CAR construction, CAR virus preparation, immune cell preparation, and phenotype detection, as well as CAR-Mo cell model and animal model construction, in vitro/in vivo CAR-Mo efficacy evaluation.
Currently, a series of CAR-Mo series products have been launched, such as CD19 CAR-Mo vector/lentivirus, lentiviral vector specially developed for CAR-Mo, and target cells for killing experiments. In addition, we can also provide customers with CAR-Mo tumor immunization services, as long as you provide the corresponding target and scFv sequence, we can build CAR-Mo vector/lentivirus, killing target cells, and can help you finish the verification of the killing function of CAR-Mo cells. Make your preclinical studies of CAR-Mo cell therapy faster and smoother!
Creative Biolabs offers integrated research and development services for CAR-Mo, encompassing five modules: preclinical candidate molecular research and development, scFv generation, in vitro/in vivo model screening, CAR-Mo engineering transformation, and quality analysis. The close connection and cooperation between these modules facilitate the rapid completion of CAR-Mo research and development services for our clients. Please feel free to contact us for more information.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION