The tumor microenvironment (TME) is a complicated ecosystem full of heterogeneity, which is mainly constituted by cancer cells, locally infiltrated immune cells, mesenchymal cells and their secreted active mediators. The TME is characterized by hypoxia and low pH, abnormal blood vessels, high permeability, inflammatory response and immunosuppression. The process of tumor formation and progression is influenced by the components of the TME through mutual and dynamic crosstalk, thus either promoting or hindering cancer progression. The TME can consistently change as the tumor progresses. Thus, it is theoretically impossible to identify the precise state of the TME. However, under certain conditions, the TME can be specialized to show typical traits.
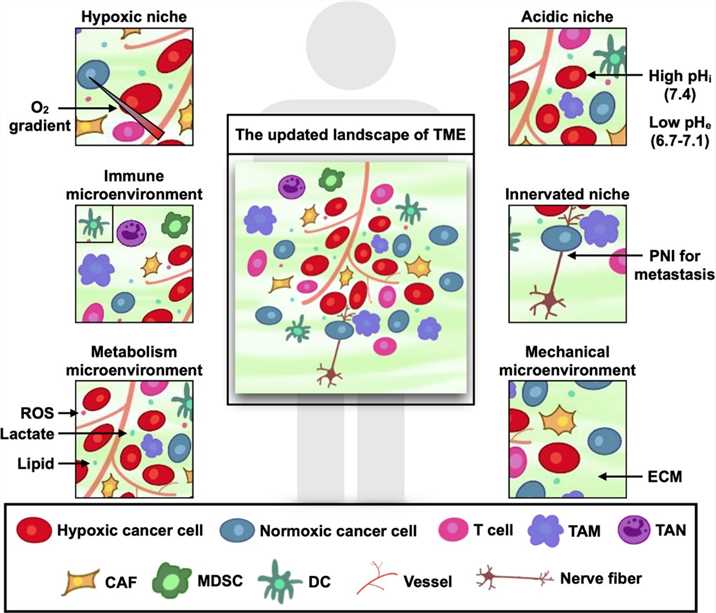 Fig.1 The updated landscape of TME.1,3
Fig.1 The updated landscape of TME.1,3
The TME has profound impacts on cancer progression and remodeling of the TME has emerged as a strategy to facilitate cancer therapy. Therapeutic strategies targeting the TME include enhancement of anti-tumor immunity, inhibition of tumor angiogenesis, administration of anti-inflammatory agents, cancer-associated fibroblasts (CAFs) depletion, targeting extracellular matrix (ECM) (e.g. collagen, hyaluronic acid depletion) and blockage of communication between tumor cells and other components in TME.
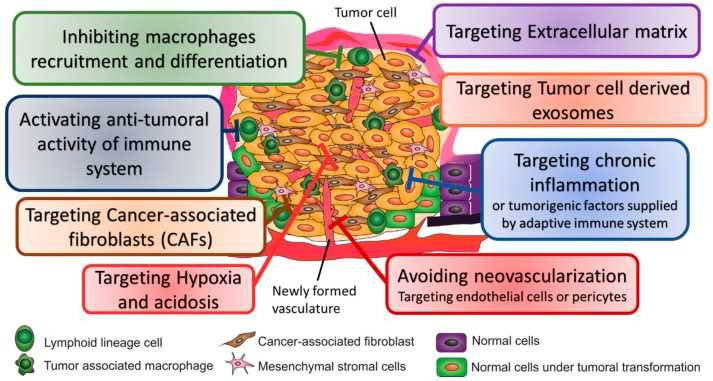 Fig.2 Strategies used to target TME for cancer therapy.2,3
Fig.2 Strategies used to target TME for cancer therapy.2,3
Immune cells play a dual role in cancer progression, that promote or hinder cancer progression. As a result, strategies to enhance anti-tumor or inhibit protumorigenic activity have been actively pursued. Major strategies are aimed at targeting immune cells and targeting the protumorigenic inflammatory pathways. In addition, because of the resistance of nonimmunogenic "cold" tumors to immunotherapy drugs, turning cold tumors into hot tumors fires up the TME and promote immune response.
Among tumor-stromal cell types, CAFs are the dominant component in the TME and play critical roles in promoting tumor progression. CAFs have emerged to be novel targets of cancer immunotherapy.
Growing tumors require the formation of new blood vessels to relieve oxygen deprivation and accumulating metabolic waste; therefore targeting angiogenesis is an attractive strategy. Antiangiogenic Normalizing or decompressing the vasculature represents a clinically translatable strategy to promote an antitumor effect. Some therapies that target proangiogenic factors such as VEGF/VEGFR2 axis not only inhibit the sprouting of new vessels but can also 'normalize' the vasculature.
The TME is characterized by disorganized tumor vasculature that results in a hypoxic microenvironment. Intratumoral hypoxia is often correlated with poor prognosis and cancer progression is often correlated with poor prognosis and cancer progression. Hypoxia-inducible factor (HIF) is a key to contributing hypoxic environment.
Nutrient limitation in the TME provides a context in which immune, stromal and cancer cells must compete for nutrients. Thus metabolic reprogramming is one of the hallmarks of cancer. Many strategies have been developed to reprogram the metabolism of tumor cells, CAFs, T cells, tumor-associated macrophages, etc.
Nowadays, TME becomes a hot area for antibody drug development and cancer treatment. Scientists at Creative Biolabs continue to understand how the TME contributes to tumorigenesis. Now we have developed a series of TME related strategies to accelerate your cancer treatment development and fasten your R&D procedure. If you are interested in TME reshaping, please feel free to contact us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION