The immune system has the functions of immune surveillance, defense and regulation. This system consists of immune organs (bone marrow, spleen, lymph nodes, etc.), immune cells (lymphocytes, phagocytes, mast cells, etc.), as well as immunoreactive substances (antibodies, lysozyme, complements, cytokines, etc.). The immune system is divided into innate immunity (also known as non-specific immunity) and adaptive immunity (also known as specific immunity), and adaptive immunity is divided into humoral immunity and cellular immunity. Creative Biolabs concludes the main types of immune cells and their respective functional roles in the immune system. Therapeutics based on these immune cells are also summarized.
Contents
Types, Locations and Functions of Immune Cells
Immune cells are commonly known as white blood cells, including lymphocytes and various phagocytes, but they can also refer to lymphocytes that can recognize antigens and produce specific immune responses. Lymphocytes are the basic components of the immune system, widely distributed in the body, mainly T lymphocytes and B lymphocytes, which are stimulated by antigen and activated to divide, proliferate and produce specific immune response. In addition to T and B lymphocytes, there are K lymphocytes and NK (natural killer) lymphocytes, a total of four lymphocyte types,among which T lymphocytes are a multifunctional group of cells. In addition to lymphocytes, the cells involved in the immune response are plasma cells, granulocytes, mast cells, antigen presenting cells and mononuclear phagocytic cells. Table 1 lists major types, locations and functions of immune cells. More detailed introductions of certain types of immune cells with significant roles are described following the table.
Table 1. Major types, locations and functions of immune cells
| Cell Type | Location and Function |
| Leukocytes | Derived from myeloid or lymphoid lineage, these are the main cells in immune system, which provide either innate or specific adaptive immunity. Myeloid cells include phagocytic, motile neutrophils, monocytes, and macrophages, providing the first-line defense against pathogens. Other myeloid cells involved in defense against parasites & in genesis of allergic reactions include eosinophils, basophils & mast cells. Lymphocytes regulate the action of other leukocytes & generate specific responses to prevent chronic/recurrent infections. |
| B cells | In mammals, B cells mature in the bone marrow, while in birds, B cells mature in the bursa of Fabricius, a lymphoid organ first discovered by Chang and Glick. They develop into antibody-secreting plasma cells. B cells express B cell receptors (BCRs) on their cell membrane that allows them to bind to specific antigen, against which it initiates a specific antibody response. |
| T cells | Originate in the bone marrow and mature in the thymus giving rise to helper, regulatory, cytotoxic T cells, or memory T cells. From the thymus, they migrate to peripheral tissues, blood, & lymphatic system. On stimulation, they secrete chemical messengers called cytokines, which stimulate the differentiation of B cells into antibody-producing cells also called plasma cells. Cytotoxic T cells in the presence of various cytokines bind to and kill infected cancer cells. |
| T helper cells | Subset of T cells, found throughout the body, with especially high titers in lymphoid organs (lymph nodes and spleen), as well as the liver, lung, blood, and the intestinal tract. T helper TH or CD4+ T cells coordinate and regulate immunological responses. TH cells mediate responses by secreting lymphokines that act on other cell types involved in mounting an immune response. |
| T cytotoxic cells | Subset of T cells, found throughout the body, with especially high titers in lymphoid organs (lymph nodes & spleen), as well as the liver, lung, blood, and the intestinal tract. T cytotoxic Tc or CD8+ T cells are involved in directly killing virus-infected cells, transplanted cells, and sometimes, eukaryotic parasites and tumor cells. CD8+ T cells have been shown to play a role in downregulating the immune response. |
| Natural Killer cells | Similar to Tc cells, NK cells directly kill certain tumors such as melanomas, lymphomas and virus-infected cells and clear herpes and cytomegalovirus-infected cells. In contrast to Tc cells, NK cells kill their target cells more effectively without the need for recognition of antigen in association with MHC molecules and are activated by secretions from TH cells. |
| Macrophages | These are phagocytic cells and function as antigen-presenting cells (APCs) as they ingest foreign materials and present these to other members of the immune system such as T cells and B cells. Besides being the initiators of an immune response, they also act as immune modulators by secreting cytokines and can also be stimulated by lymphokines, to exhibit increased levels of phagocytosis. |
| Dendritic cells | Originate in the bone marrow and form another class of APCs. These are found in the lymphoid organs such as the thymus, lymph nodes, and spleen along with the bloodstream and other tissues. They function to capture and process antigens in lymphoid organs at the time of initiation of an immune response. |
| Neutrophils | These cells, which ingest, kill, and digest pathogens, are the most highly adherent and motile phagocytic leukocytes, and the first cell types to be recruited to acute inflammatory sites. Their functions are dependent on adherence molecule CD11b/CD18, or biochemical pathways, such as the respiratory burst associated with cytochrome b558. |
| Eosinophils | Defend against parasites and participate in hypersensitivity reactions. Their cytotoxicity is mediated through cytoplasmic granules containing eosinophilic basic and cationic proteins. |
| Basophils | Along with their tissue counterparts, mast cells produce cytokines required for defense against parasites and allergic inflammation. They display surface membrane receptors for IgE antibodies and possess cytoplasmic granules containing heparin and histamine. When cell-bound IgE antibodies are cross linked by antigens, the eosinophils degranulate releasing low-molecular weight vasoactive mediators (e.g., histamine), which mediate their biological effects. |
| Monocytes/macrophages | These are involved in phagocytosis and intracellular killing of microorganisms. Macrophages are differentiated monocytes, residing in the reticuloendothelial systems and act as antigen-presenting cells presenting processed peptides to T cells. They are recruited to inflammatory sites and further activated by exposure to certain cytokines, which potentiate their biologic functions. |
 T cells are also known as thymus dependent lymphocytes, which come from pluripotent stem cells from bone marrow (in embryonic stage come from yolk sac and liver). The pluripotent stem cells migrate into the thymus and differentiates into immune T cells induced by thymic hormones. T cells are a kind of cells with the largest number and the most complex function in lymphocytes. According to its function, it can be divided into three main subgroups: helper T cells, inhibitory T cells and cytotoxic T cells. T cells are the main components of lymphocytes, which have a variety of biological functions, such as directly killing target cells, assisting or inhibiting B cells to produce antibodies, responding to specific antigens and mitogen, and producing cytokines, which are significant for the body to resist disease infection, tumor formation of heroic fighters. The immune response produced by T cells is cellular immunity. There are two main forms of cellular immunity: one is specific binding to target cells, destruction of target cell membrane and direct killing of target cells; the other is the release of lymphoid factor, which ultimately expands and enhances the immune effect.
T cells are also known as thymus dependent lymphocytes, which come from pluripotent stem cells from bone marrow (in embryonic stage come from yolk sac and liver). The pluripotent stem cells migrate into the thymus and differentiates into immune T cells induced by thymic hormones. T cells are a kind of cells with the largest number and the most complex function in lymphocytes. According to its function, it can be divided into three main subgroups: helper T cells, inhibitory T cells and cytotoxic T cells. T cells are the main components of lymphocytes, which have a variety of biological functions, such as directly killing target cells, assisting or inhibiting B cells to produce antibodies, responding to specific antigens and mitogen, and producing cytokines, which are significant for the body to resist disease infection, tumor formation of heroic fighters. The immune response produced by T cells is cellular immunity. There are two main forms of cellular immunity: one is specific binding to target cells, destruction of target cell membrane and direct killing of target cells; the other is the release of lymphoid factor, which ultimately expands and enhances the immune effect.
 B lymphocytes are also referred to as B cells, which are derived from pluripotent stem cells of bone marrow. Compared with T cells, B cells are slightly larger. Mature B cells migrate out of peripheral blood and enter spleen and lymph nodes, which are mainly distributed in splenic nodules, splenic cord, lymphoid nodules, lymphatic cords and submucosal lymphoid nodules of digestive tract. Stimulated by antigen, they differentiate and proliferate into a large number of plasma cells, synthesize and secrete antibodies and circulate in the blood, thus give full play to the function of humoral immunity. Most vaccines used today produce antibodies by stimulating these B lymphocytes. The number of B cells in bone marrow and collecting lymph nodes is higher than that in blood and lymph nodes, and even less in thoracic ducts. Only a few of them participate in recirculation. There are many different markers on the cell membrane of B cells, mainly surface antigens and surface receptors. These surface markers are giant protein molecules that bind to the cell membrane. B1 cells are T cell-independent cells, while B2 is T cell-dependent. B cells survive for a short period of time in the body, only a few days to a few weeks, but their memory cells can exist for a long time.
B lymphocytes are also referred to as B cells, which are derived from pluripotent stem cells of bone marrow. Compared with T cells, B cells are slightly larger. Mature B cells migrate out of peripheral blood and enter spleen and lymph nodes, which are mainly distributed in splenic nodules, splenic cord, lymphoid nodules, lymphatic cords and submucosal lymphoid nodules of digestive tract. Stimulated by antigen, they differentiate and proliferate into a large number of plasma cells, synthesize and secrete antibodies and circulate in the blood, thus give full play to the function of humoral immunity. Most vaccines used today produce antibodies by stimulating these B lymphocytes. The number of B cells in bone marrow and collecting lymph nodes is higher than that in blood and lymph nodes, and even less in thoracic ducts. Only a few of them participate in recirculation. There are many different markers on the cell membrane of B cells, mainly surface antigens and surface receptors. These surface markers are giant protein molecules that bind to the cell membrane. B1 cells are T cell-independent cells, while B2 is T cell-dependent. B cells survive for a short period of time in the body, only a few days to a few weeks, but their memory cells can exist for a long time.
 Natural killer (NK) cells are derived from bone marrow lymphoid stem cells. Their differentiation and development depend on bone marrow and thymus microenvironment. They are mainly distributed in bone marrow, peripheral blood, liver, spleen, lung and lymph nodes. Different from T and B cells, NK cells are a kind of lymphocytes which can kill tumor cells and virus infected cells without pre-sensitization. The killing activity of NK cells is not limited by major histocompatibility complex (MHC) and does not depend on antibodies, thus it is called natural killing activity. NK cells are rich in cytoplasm and contain large azurophilic granules. The content of granules is positively correlated with the killing activity of NK cells. The target cells of NK cells are mainly some tumor cells (including some cell lines), virus infected cells, some self-tissue cells (such as blood cells), parasites and so on. Therefore, NK cells are important immune factors for anti-tumor and anti-infection. It is also involved in type II hypersensitivity and graft-versus-host response. The main killing mediators of NK cells are perforin, NK cytotoxic factors and tumor necrosis factors (TNFs).
Natural killer (NK) cells are derived from bone marrow lymphoid stem cells. Their differentiation and development depend on bone marrow and thymus microenvironment. They are mainly distributed in bone marrow, peripheral blood, liver, spleen, lung and lymph nodes. Different from T and B cells, NK cells are a kind of lymphocytes which can kill tumor cells and virus infected cells without pre-sensitization. The killing activity of NK cells is not limited by major histocompatibility complex (MHC) and does not depend on antibodies, thus it is called natural killing activity. NK cells are rich in cytoplasm and contain large azurophilic granules. The content of granules is positively correlated with the killing activity of NK cells. The target cells of NK cells are mainly some tumor cells (including some cell lines), virus infected cells, some self-tissue cells (such as blood cells), parasites and so on. Therefore, NK cells are important immune factors for anti-tumor and anti-infection. It is also involved in type II hypersensitivity and graft-versus-host response. The main killing mediators of NK cells are perforin, NK cytotoxic factors and tumor necrosis factors (TNFs).
 Macrophage (Mø) is a kind of white blood cell in tissue, which comes from monocytes, which in turn come from progenitor cells in bone marrow. Macrophages and monocytes are phagocytes and are involved in non-specific defense (innate immunity) and specific defense (cellular immunity) in vertebrates. Their main function is to phage cell fragments and pathogens (that is, phagocytosis and digestion) in the form of fixed cells or free cells, and activate lymphocytes or other immune cells to respond to pathogens. Macrophages are amoeba-like cells that devour and treat large foreign bodies, old waste excreted by cells, red blood cells at the end of their lives, and go to the site of inflammation to deal with foreign bodies. Macrophages are a kind of white blood cells with a wide range of protection, which exist not only in the blood, but also in the whole body. Depending on the location, the name and shape vary. Monocytes circulate to the whole body with the blood and run to the inflammatory site. The defense system of the single-celled biological age is quite simple, with macrophages phagocytosis of foreign bodies, making it become garbage and excrete out of the body.
Macrophage (Mø) is a kind of white blood cell in tissue, which comes from monocytes, which in turn come from progenitor cells in bone marrow. Macrophages and monocytes are phagocytes and are involved in non-specific defense (innate immunity) and specific defense (cellular immunity) in vertebrates. Their main function is to phage cell fragments and pathogens (that is, phagocytosis and digestion) in the form of fixed cells or free cells, and activate lymphocytes or other immune cells to respond to pathogens. Macrophages are amoeba-like cells that devour and treat large foreign bodies, old waste excreted by cells, red blood cells at the end of their lives, and go to the site of inflammation to deal with foreign bodies. Macrophages are a kind of white blood cells with a wide range of protection, which exist not only in the blood, but also in the whole body. Depending on the location, the name and shape vary. Monocytes circulate to the whole body with the blood and run to the inflammatory site. The defense system of the single-celled biological age is quite simple, with macrophages phagocytosis of foreign bodies, making it become garbage and excrete out of the body.
 Dendritic cells (DCs), discovered by Canadian scholar Steinman in 1973, are the most powerful antigen-presenting cells (APCs), named after many dendritic or pseudopod processes when they mature. Dendritic cells have no specific cell surface molecular markers, which are mainly identified by morphology, combinatorial cell surface markers, activation of initial T cells in mixed lymphocyte reaction and so on. DCs are originated from bone marrow pluripotent hematopoietic stem cells. There are two main ways of DC differentiation: (1) myeloid stem cells differentiate into DC, under the stimulation of GM-CSF, which is called myeloid DC (MDC), also known as DC1; (2) derived from lymphoid stem cells, called lymphoid DC (LDC) or plasma cell-like DC (pDC), also known as DC2. DCs are widely distributed in the skin, airway, lymphoid organs and other parts, with a high degree of heterogeneity. Most of DCs in human body are in immature state, expressing low levels of costimulatory factors and adhesion factors, and the ability to stimulate the proliferation of allogeneic mixed lymphocytes is low, but immature DCs have strong antigen phagocytosis. When ingested antigens or stimulated by some factors, the immature DCs differentiated into mature DCs which expressed high levels of costimulatory factors and adhesion factors. As the most powerful APCs, they can induce specific cytotoxic T lymphocyte (CTL) production.
Dendritic cells (DCs), discovered by Canadian scholar Steinman in 1973, are the most powerful antigen-presenting cells (APCs), named after many dendritic or pseudopod processes when they mature. Dendritic cells have no specific cell surface molecular markers, which are mainly identified by morphology, combinatorial cell surface markers, activation of initial T cells in mixed lymphocyte reaction and so on. DCs are originated from bone marrow pluripotent hematopoietic stem cells. There are two main ways of DC differentiation: (1) myeloid stem cells differentiate into DC, under the stimulation of GM-CSF, which is called myeloid DC (MDC), also known as DC1; (2) derived from lymphoid stem cells, called lymphoid DC (LDC) or plasma cell-like DC (pDC), also known as DC2. DCs are widely distributed in the skin, airway, lymphoid organs and other parts, with a high degree of heterogeneity. Most of DCs in human body are in immature state, expressing low levels of costimulatory factors and adhesion factors, and the ability to stimulate the proliferation of allogeneic mixed lymphocytes is low, but immature DCs have strong antigen phagocytosis. When ingested antigens or stimulated by some factors, the immature DCs differentiated into mature DCs which expressed high levels of costimulatory factors and adhesion factors. As the most powerful APCs, they can induce specific cytotoxic T lymphocyte (CTL) production.
 Mast cells come from hematopoietic stem cells, but in fact, mast cells that have just entered the peripheral circulatory system from the bone marrow are still immature, only when their precursor cells migrate to the place where they eventually settle. That is, differentiation and/or maturation can only be completed in vascular tissue or serous cavity. Mast cells are widely distributed around the microvessels under the skin and visceral mucosa, which are often exposed to pathogens, allergens and other environmental substances. Mast cells secrete a variety of cytokines, express MHC and B7 molecules and IgE Fc receptors, and release allergic mediators. They also behave weak phagocytosis. The distribution and function characteristics of mast cells make them together with dendritic cells become the first cell groups in the hematopoietic-immune system to interact with allergens, other antigens and invasive pathogens in the hematopoietic-immune system.
Mast cells come from hematopoietic stem cells, but in fact, mast cells that have just entered the peripheral circulatory system from the bone marrow are still immature, only when their precursor cells migrate to the place where they eventually settle. That is, differentiation and/or maturation can only be completed in vascular tissue or serous cavity. Mast cells are widely distributed around the microvessels under the skin and visceral mucosa, which are often exposed to pathogens, allergens and other environmental substances. Mast cells secrete a variety of cytokines, express MHC and B7 molecules and IgE Fc receptors, and release allergic mediators. They also behave weak phagocytosis. The distribution and function characteristics of mast cells make them together with dendritic cells become the first cell groups in the hematopoietic-immune system to interact with allergens, other antigens and invasive pathogens in the hematopoietic-immune system.
 Neutrophils are colorless or light red cytoplasm in Wright's stained blood smears. Neutrophils, which come from bone marrow and have lobulated or rod-shaped nuclei, have chemotaxis, phagocytosis and bactericidal effect. The cytoplasm contains a large number of neutral fine particles that are neither basophilic nor eosinophilic. Most of these particles are lysosomes, which contain rich enzymes such as myeloperoxidase, lysozyme, alkaline phosphatase and acid hydrolase, and they are related to the phagocytosis and digestion of cells.
Neutrophils are colorless or light red cytoplasm in Wright's stained blood smears. Neutrophils, which come from bone marrow and have lobulated or rod-shaped nuclei, have chemotaxis, phagocytosis and bactericidal effect. The cytoplasm contains a large number of neutral fine particles that are neither basophilic nor eosinophilic. Most of these particles are lysosomes, which contain rich enzymes such as myeloperoxidase, lysozyme, alkaline phosphatase and acid hydrolase, and they are related to the phagocytosis and digestion of cells.
Immune Cell-based Therapies
Based on the significant roles of T cells in the immune system, many small-molecule drugs targeting T cells and T-cell based immunotherapies have been developed for the treatment of intractable diseases including autoimmune diseases and cancer. T-cell based immunotherapies mainly utilize the mechanisms of T cell-mediated immune responses and the effects of some other immune cells such as DCs, natural killer (NK) cells, and macrophages. Currently developed T cell-based immunotherapies mainly include monoclonal antibodies targeting T cells, adoptive transfer of genetically engineered T cells, and T cell vaccine.
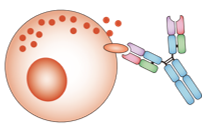
 Both health cells (including immune cells, especially T cells) and tumor cells express multiple receptor and/or ligand molecules on their membrane and into the tumor microenvironment. Several of these molecules participate in pairs in stimulating or inhibiting immune progress, which are known as immune checkpoints. When immune system is attacking pathogens, these immune checkpoint molecules can protect the normal tissues from damage. The cancer cells cleverly escape from immune attack by dysregulating immune checkpoint related proteins. This has led to the development of several checkpoint-targeted small-molecule and monoclonal antibody drugs that are currently being tested in clinical trials or have been approved for a number of cancers. Small molecule drugs have desirable benefits over monoclonal antibodies, including greater cell permeability, organ specificity, longer half-lives, cheaper production costs, and the possibility for oral administration. Currently developing small molecule inhibitors targeting immune checkpoints and the related cellular pathways are listed in Table 2.
Both health cells (including immune cells, especially T cells) and tumor cells express multiple receptor and/or ligand molecules on their membrane and into the tumor microenvironment. Several of these molecules participate in pairs in stimulating or inhibiting immune progress, which are known as immune checkpoints. When immune system is attacking pathogens, these immune checkpoint molecules can protect the normal tissues from damage. The cancer cells cleverly escape from immune attack by dysregulating immune checkpoint related proteins. This has led to the development of several checkpoint-targeted small-molecule and monoclonal antibody drugs that are currently being tested in clinical trials or have been approved for a number of cancers. Small molecule drugs have desirable benefits over monoclonal antibodies, including greater cell permeability, organ specificity, longer half-lives, cheaper production costs, and the possibility for oral administration. Currently developing small molecule inhibitors targeting immune checkpoints and the related cellular pathways are listed in Table 2.
Table 2. Current small molecule inhibitors targeting immune checkpoints and cellular pathways
| Immune Checkpoint | Small Molecule Inhibitors | Cell Pathways |
| PD-1/PD-L1 | BMS-8, 37, 202, 230, 242, 1001, 1166, SB415286*, vorinostat*, panobinostat*, decitabine*, entitostat*, JQ1*, I-BET151*, GSK503* | Invasion, Angiogenesis, T-Cell suppression, |
| CTLA4 | Compounds "8 and 9", entitostat*, panobinostat*, ACY-241*, azacytidine* | Angiogenesis, T-Cell activation, Cell signaling |
| OX40 | DB36, DB71, DB15, CVN, MGCD0103*, SNDX-275*, azacytidine* | Angiogenesis, Cell activation, Tumor necrosis |
| LAG-3 | IMP32, BMS986016 | T-Cell proliferation, Cell maturation & activation |
| TIM-3 | TSR-022, Sym023, ATIK2a | Tumorigenesis, Cell invasion & activation |
| B7-H3 | c-MYC SMIs, vorinostat*, DZNep* | Migration, Angiogenesis, Cellular cycle & signaling |
*, Epigenetic SMI.
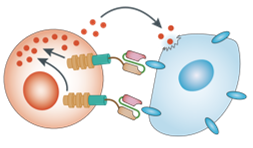
 Monoclonal antibody (mAb)-based treatment of cancer has been established as one of the most successful therapeutic strategies for both hematologic malignancies and solid tumors in the last decades. The main desired mechanism of action is engagement with cell surface receptors (to either activate or inhibit signaling), or to activate/mediate antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), complement-dependent cell-mediated cytotoxicity (CDCC), and programmed cell death (PCD). Most of the monoclonal antibodies are designed to target various antigens existing in the tumor microenvironment, including tumor antigens, cytokines, immune checkpoints, and marker molecules expressed on the membrane of immune cells. As a group of immune cell surface glycoproteins with a multitude of functions, the clusters of differentiation (CD antigens) provide the interface between a cell and the external environment that includes other cells. Serving as receptors and ligands, CD antigens regulate cell signaling and immune response, making them popular in therapeutic antibody development.
Monoclonal antibody (mAb)-based treatment of cancer has been established as one of the most successful therapeutic strategies for both hematologic malignancies and solid tumors in the last decades. The main desired mechanism of action is engagement with cell surface receptors (to either activate or inhibit signaling), or to activate/mediate antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), antibody-dependent cellular phagocytosis (ADCP), complement-dependent cell-mediated cytotoxicity (CDCC), and programmed cell death (PCD). Most of the monoclonal antibodies are designed to target various antigens existing in the tumor microenvironment, including tumor antigens, cytokines, immune checkpoints, and marker molecules expressed on the membrane of immune cells. As a group of immune cell surface glycoproteins with a multitude of functions, the clusters of differentiation (CD antigens) provide the interface between a cell and the external environment that includes other cells. Serving as receptors and ligands, CD antigens regulate cell signaling and immune response, making them popular in therapeutic antibody development.
 Adoptive cell therapy (ACT) is a highly personalized cancer therapy that involves administration to the cancer-bearing host of immune cells with direct anticancer activity. ACT has multiple advantages compared with other forms of cancer immunotherapy that rely on the active in vivo development of sufficient numbers of antitumor T cells with the functions necessary to mediate cancer regression. For use in ACT, large numbers of immune cells including anti-tumor lymphocytes can be readily grown in vitro and selected for high-avidity recognition of the tumor, as well as for the effector functions required to mediate cancer regression. In vitro activation allows such cells to be released from the inhibitory factors that exist in vivo. Perhaps most importantly, ACT enables the manipulation of the host before cell transfer to provide a favorable microenvironment that better supports antitumor immunity. ACT is a "living" treatment because the administered cells can proliferate in vivo and maintain their antitumor effector functions.
Adoptive cell therapy (ACT) is a highly personalized cancer therapy that involves administration to the cancer-bearing host of immune cells with direct anticancer activity. ACT has multiple advantages compared with other forms of cancer immunotherapy that rely on the active in vivo development of sufficient numbers of antitumor T cells with the functions necessary to mediate cancer regression. For use in ACT, large numbers of immune cells including anti-tumor lymphocytes can be readily grown in vitro and selected for high-avidity recognition of the tumor, as well as for the effector functions required to mediate cancer regression. In vitro activation allows such cells to be released from the inhibitory factors that exist in vivo. Perhaps most importantly, ACT enables the manipulation of the host before cell transfer to provide a favorable microenvironment that better supports antitumor immunity. ACT is a "living" treatment because the administered cells can proliferate in vivo and maintain their antitumor effector functions.
ACT has used either natural host cells that exhibit antitumor reactivity or host cells that have been genetically engineered with anti-tumor T cell receptors (TCRs) or chimeric antigen receptors (CARs). With the use of these approaches, ACT has mediated dramatic regressions in a variety of cancer histologies, including melanoma, cervical cancer, lymphoma, leukemia, bile duct cancer, and neuroblastoma. For CAR-T cell therapy, while the mechanism of action is not fully understood, evidence suggests that CAR-T cell therapies stimulate a T cell response against antigen-expressing cells, including normal and malignant cells. The external targeting domain binds to the antigen, activating the CAR-T cell. Once activated, CAR-T cells release cytokines and other soluble mediators that may directly kill antigen-expressing target cells and normal cells. Two autologous CAR-T cell therapies (Kymriah™ and Yescarta™) have been approved by the Food and Drug Administration (FDA) and the Europe Union. Kymriah™ is for the treatment of pediatric patients and young adults with refractory or relapse (R/R) B cell precursor acute lymphoblastic leukemia and Yescarta™ is for the treatment of adult patients with R/R large B cell lymphoma. In common, both are CD19-specific CAR-T cell therapies lysing CD19-positive targets.
Current develop of cancer therapies that based on adoptive transfer of genetically engineered immune cells mainly focus on T cells, NK cells, macrophages, and dendritic cells. Currently developed and developing adoptive cell therapies based on CAR/TCR-modified immune cells mainly are CAR-T, TCR-T, CAR-NK, CAR-NKT, and CAR-Macrophage. Table 4 lists some of these adoptive cell therapies in clinical trials.
Table 4 . Clinical trials of adoptive cell therapies based on CAR/TCR-modified immune cells
| Type | Nct_id | Title | Condition | Status | Lead_sponsor | Study_first_posted |
| CAR-T | NCT03423992 | Personalized Chimeric Antigen Receptor T Cell Immunotherapy for Patients With Recurrent Malignant Gliomas | Glioma, Malignant Glioma of Brain, Recurrence Tumor | Recruiting | Xuanwu Hospital, Beijing | February 6, 2018 |
| CAR-T | NCT03758417 | A Study of LCAR-B38M CAR-T Cells, a Chimeric Antigen Receptor T-cell (CAR-T) Therapy Directed Against B-cell Maturation Antigen (BCMA) in Chinese Participants With Relapsed or Refractory Multiple Myeloma | Multiple Myeloma | Recruiting | Nanjing Legend Biotech Co. | November 29, 2018 |
| CAR-T | NCT03874897 | Chimeric Antigen Receptor T Cells Targeting claudin18.2 in Solid Tumors. | Advanced Solid Tumor | Recruiting | Peking University | March 14, 2019 |
| CAR-T | NCT03938987 | Anti-CD19, Dual Co-stimulatory (4-1BB, CD3ζ) Chimeric Antigen Receptor T-cells in Patients With Relapsed/Refractory Aggressive Lymphoma or Acute Lymphoblastic Leukemia (ALL) | Relapsed Non Hodgkin Lymphoma, Relapsed Adult ALL, Relapsed Pediatric ALL | Not yet recruiting | University of Alberta | May 6, 2019 |
| CAR-T | NCT03241940 | Phase I CD19/CD22 Chimeric Antigen Receptor T Cells in Peds Recurrent/Refractory B Cell Malignancies | B Acute Lymphoblastic Leukemia, CD19 Positive, Minimal Residual Disease, Philadelphia Chromosome Positive, Recurrent Adult Acute Lymphoblastic Leukemia, Recurrent Childhood Acute Lymphoblastic Leukemia, Refractory Acute Lymphoblastic Leukemia | Recruiting | Crystal Mackall, MD | August 8, 2017 |
| CAR-T | NCT03884751 | Chimeric Antigen Receptor T Cells Targeting Glypican-3 | Hepatocellular Carcinoma | Not yet recruiting | Carsgen Therapeutics, Ltd. | March 21, 2019 |
| CAR-T | NCT03434769 | AntiCD19 Chimeric Antigen Receptor T Cells for Relapsed or Refractory Non Hodgkin Lymphoma | Non-Hodgkin Lymphoma | Recruiting | Case Comprehensive Cancer Center | February 15, 2018 |
| CAR-T | NCT02442297 | T Cells Expressing HER2-specific Chimeric Antigen Receptors(CAR) for Patients With HER2-Positive CNS Tumors | Brain Tumor, Recurrent, Brain Tumor, Refractory | Recruiting | Baylor College of Medicine | May 13, 2015 |
| CAR-T | NCT03233854 | Phase I CD19/CD22 Chimeric Antigen Receptor(CAR) T Cells in Adults With Recurrent/Refractory B Cell Malignancies | B Acute Lymphoblastic Leukemia, CD19 Positive, Diffuse Large B-Cell Lymphoma Associated With Chronic Inflammation, Diffuse Large B-Cell Lymphoma, Not Otherwise Specified, Epstein-Barr Virus Positive Diffuse Large B-Cell Lymphoma of the Elderly, Minimal Residual Disease, Philadelphia Chromosome Positive, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Mediastinal (Thymic) Large B-Cell Cell Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Mediastinal (Thymic) Large B-Cell Cell Lymphoma, T-Cell/Histiocyte-Rich Large B-Cell Lymphoma | Recruiting | Crystal Mackall, MD | July 31, 2017 |
| CAR-T | NCT03118180 | CD19 Targeted Chimeric Antigen Receptor T Cells for B Cell Lymphoma | Lymphoma | Recruiting | Zhejiang University | April 18, 2017 |
| CAR-T | NCT02992834 | Anti-CD19:TCRζ Chimeric Antigen Receptor-T Cells in the Treatment for CD19+ B Cell Lymphoma | Lymphoma, B Cell | Not yet recruiting | jiangjingting | December 14, 2016 |
| CAR-T | NCT02349698 | A Clinical Research of CAR-T Cells Targeting CD19 Positive Malignant B-cell Derived Leukemia and Lymphoma | Leukemia, Lymphoma | Recruiting | Southwest Hospital, China | January 29, 2015 |
| CAR-T | NCT03579888 | CD19+ Specific Chimeric Antigen Receptor T Cells in Treating Participants With CD19+ Lymphoid Malignancies | Acute Lymphoblastic Leukemia, Blasts More Than 5 Percent of Bone Marrow Nucleated Cells, Blasts More Than 5 Percent of Peripheral Blood White Cells, CD19 Positive, Chronic Lymphocytic Leukemia, Minimal Residual Disease, Non-Hodgkin Lymphoma, Small Lymphocytic Lymphoma | Not yet recruiting | M.D. Anderson Cancer Center | July 9, 2018 |
| CAR-T | NCT03624686 | Production of Clinical-grade Anti-CD19 Chimeric Antigen Receptor T Cells for Refractory B-cell Malignancies | Leukemia | Recruiting | National Taiwan University Hospital | August 10, 2018 |
| CAR-T | NCT02659943 | T Cells Expressing a Fully-human AntiCD19 Chimeric Antigen Receptor for Treating B-cell Malignancies | Lymphoma, B-Cell, Lymphoma, Non-hodgkins | Active, not recruiting | National Cancer Institute (NCI) | January 21, 2016 |
| CAR-T | NCT03291444 | CAR-T Cells Combined With Peptide Specific Dendritic Cell in Relapsed/Refractory Leukemia/MDS | Leukemia, Acute Lymphocytic (ALL), Leukemia, Acute Myelogenous (AML), Myelodysplastic Syndromes | Recruiting | Zhujiang Hospital | September 25, 2017 |
| CAR-T | NCT04082910 | Metoprolol for the Treatment of Cytokine Release Syndrome in Patients Treated With Chimeric Antigen Receptor T Cells | Solid Tumor, Hematologial Malignancy | Recruiting | Chinese PLA General Hospital | September 10, 2019 |
| CAR-T | NCT04049513 | ENABLE (Engaging Toll-like Receptor Signalling for B-cell Lymphoma Chimeric Antigen Receptor Therapy) | Lymphomas Non-Hodgkin's B-Cell, Diffuse Large B-cell Lymphoma (DLBCL), Primary Mediastinal B-cell Lymphoma (PMBCL), Transformed Follicular Lymphoma (TFL), Follicular Lymphoma (FL), Mantle Cell Lymphoma (MCL) | Not yet recruiting | Malaghan Institute of Medical Research | August 8, 2019 |
| CAR-T | NCT03086954 | Study Evaluating the Efficacy and Safety With CAR-T Immunotherapy for CD19 Positive Lymphoma | Lymphoma | Not yet recruiting | Sinobioway Cell Therapy Co., Ltd. | March 22, 2017 |
| CAR-T | NCT03890198 | A Phase 1 Study of LCAR-C182A/LCAR-C182B/LCAR-C182C Cells,a Chimeric Antigen Receptor T-cell (CAR-T) | Gastric Cancer, Pancreatic Ductal Adenocarcinoma | Recruiting | First Affiliated Hospital Xi'an Jiaotong University | March 26, 2019 |
| CAR-T | NCT01822652 | 3rd Generation GD-2 Chimeric Antigen Receptor and iCaspase Suicide Safety Switch, Neuroblastoma, GRAIN | Neuroblastoma | Active, not recruiting | Baylor College of Medicine | April 2, 2013 |
| CAR-T | NCT04029038 | Modified Immune Cells (CD19-CD22 CAR-T Cells) in Treating Patients With Recurrent or Refractory CD19 Positive, CD22 Positive Leukemia or Lymphoma | CD19 Positive, CD22 Positive, Minimal Residual Disease, Progressive Disease, Recurrent B Acute Lymphoblastic Leukemia, Recurrent Chronic Lymphocytic Leukemia, Recurrent Non-Hodgkin Lymphoma, Refractory B Acute Lymphoblastic Leukemia, Refractory Chronic Lymphocytic Leukemia, Refractory Non-Hodgkin Lymphoma | Not yet recruiting | M.D. Anderson Cancer Center | July 23, 2019 |
| CAR-T | NCT03980288 | 4th Generation Chimeric Antigen Receptor T Cells Targeting Glypican-3 | Advanced Hepatocellular Carcinoma | Not yet recruiting | Zhejiang University | June 10, 2019 |
| CAR-T | NCT03327285 | C-CAR011 Treatment in Subjects With ALL After HSCT | Acute Lymphoblastic Leukemia(ALL) | Not yet recruiting | Peking University People's Hospital | October 31, 2017 |
| CAR-T | NCT02813837 | Chimeric Antigen Receptor T Cells (CART) Therapy in Refractory/Relapsed B Cell Hematologic Malignancies | Leukemia, B-Cell, Lymphoma, B-Cell | Recruiting | Innovative Cellular Therapeutics Co., Ltd. | June 27, 2016 |
| CAR-T | NCT02259556 | CD30-directed Chimeric Antigen Receptor T (CART30) Therapy in Relapsed and Refractory CD30 Positive Lymphomas | Hodgkin's Lymphoma, Non-Hodgkin's Lymphoma | Recruiting | Chinese PLA General Hospital | October 8, 2014 |
| CAR-T | NCT03971799 | Study of Anti-CD33 Chimeric Antigen Receptor-Expressing T Cells (CD33CART) in Children and Young Adults With Relapsed/Refractory Acute Myeloid Leukemia | Acute Myelogenous Leukemia | Not yet recruiting | Center for International Blood and Marrow Transplant Research | June 3, 2019 |
| CAR-T | NCT01087294 | Administration of Anti-CD19-chimeric-antigen-receptor-transduced T Cells From the Original Transplant Donor to Patients With Recurrent or Persistent B-cell Malignancies After Allogeneic Stem Cell Transplantation | Leukemia, B-cell, Lymphoma, Hodgkins, Lymphoma, Non-hodgkins, Lymphoma, B-Cell | Recruiting | National Cancer Institute (NCI) | March 16, 2010 |
| CAR-T | NCT03076437 | Anti-CD19 Chimeric Antigen Receptor (CAR)-Transduced T Cell Therapy for Patients With B Cell Malignancies | Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Lymphoma | Recruiting | Shenzhen Institute for Innovation and Translational Medicine | March 10, 2017 |
| CAR-T | NCT02905188 | Glypican 3-specific Chimeric Antigen Receptor Expressing T Cells for Hepatocellular Carcinoma (GLYCAR) | Hepatocellular Carcinoma | Recruiting | Baylor College of Medicine | September 19, 2016 |
| CAR-T | NCT02932956 | Glypican 3-specific Chimeric Antigen Receptor Expressed in T Cells for Patients With Pediatric Solid Tumors (GAP) | Liver Cancer Pediatric | Recruiting | Baylor College of Medicine | October 13, 2016 |
| CAR-T | NCT02456350 | Anti-CD19 Chimeric Antigen Receptor (CAR)-Transduced T Cell Therapy for Patients With B Cell Malignancies | Chronic Lymphocytic Leukemia, Acute Lymphocytic Leukemia, Lymphoma | Recruiting | Shenzhen Second People's Hospital | May 28, 2015 |
| CAR-T | NCT03049449 | T Cells Expressing a Fully-Human Anti-CD30 Chimeric Antigen Receptor for Treating CD30-Expressing Lymphomas | Lymphoma, Large-Cell, Anaplasitc, Hodgkin Disease, Lymphoma, Hodgkins, Enteropathy-Associated T-Cell Lymphoma | Active, not recruiting | National Cancer Institute (NCI) | February 10, 2017 |
| CAR-T | NCT04093648 | T Cells co- Expressing a Second Generation Glypican 3-specific Chimeric Antigen Receptor With Cytokines Interleukin-21 and 15 as Immunotherapy for Patients With Liver Cancer (TEGAR) | Hepatocellular Carcinoma, Hepatoblastoma | Not yet recruiting | Baylor College of Medicine | September 18, 2019 |
| CAR-T | NCT03548207 | A Study of JNJ-68284528, a Chimeric Antigen Receptor T Cell (CAR-T) Therapy Directed Against B-Cell Maturation Antigen (BCMA) in Participants With Relapsed or Refractory Multiple Myeloma | Multiple Myeloma | Recruiting | Janssen Research & Development, LLC | June 7, 2018 |
| CAR-T | NCT03302403 | Clinical Study of Redirected Autologous T Cells With a Chimeric Antigen Receptor in Patients With Malignant Tumors | B Cell Lymphoma, B Cell Leukemia, Myeloma, Hepatocellular Carcinoma, Pancreatic Carcinoma, Adenocarcinoma of Esophagogastric Junction | Not yet recruiting | Kang YU | October 5, 2017 |
| CAR-T | NCT03696030 | HER2-CAR T Cells in Treating Patients With Recurrent Brain or Leptomeningeal Metastases | HER2/Neu Positive, Malignant Neoplasm, Metastatic Malignant Neoplasm in the Brain, Metastatic Malignant Neoplasm in the Leptomeninges | Recruiting | City of Hope Medical Center | October 4, 2018 |
| CAR-T | NCT00902044 | Her2 Chimeric Antigen Receptor Expressing T Cells in Advanced Sarcoma | Sarcoma | Recruiting | Baylor College of Medicine | May 14, 2009 |
| CAR-T | NCT03500991 | HER2-specific CAR-T Cell Locoregional Immunotherapy for HER2-positive Recurrent/Refractory Pediatric CNS Tumors | Central Nervous System Tumor, Pediatric, Glioma, Ependymoma, Medulloblastoma, Germ Cell Tumor, Atypical Teratoid/Rhabdoid Tumor, Primitive Neuroectodermal Tumor, Choroid Plexus Carcinoma, Pineoblastoma | Recruiting | Seattle Children's Hospital | April 17, 2018 |
| CAR-T | NCT03144583 | Pilot Study on the Infusion of ARI-0001 Cells in Patients With CD19+ Leukemia or Lymphoma Refractory to Therapy | Leukemia, Lymphoma | Active, not recruiting | Sara V. Latorre | May 9, 2017 |
| CAR-T | NCT03448978 | Autologous CD8+ T-cells Expressing an Anti-BCMA CAR in Patients With Myeloma | Multiple Myeloma | Recruiting | Cartesian Therapeutics | February 28, 2018 |
| CAR-T | NCT03277729 | A Phase I/II Study to Evaluate the Safety of Cellular Immunotherapy Using Autologous T Cells Engineered to Express a CD20-Specific Chimeric Antigen Receptor for Patients With Relapsed or Refractory B Cell Non-Hodgkin Lymphomas | CD20 Positive, Recurrent B-Cell Non-Hodgkin Lymphoma, Recurrent Chronic Lymphocytic Leukemia, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Follicular Lymphoma, Recurrent Lymphoplasmacytic Lymphoma, Recurrent Mantle Cell Lymphoma, Recurrent Marginal Zone Lymphoma, Refractory B-Cell Non-Hodgkin Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Follicular Lymphoma, Refractory Lymphoplasmacytic Lymphoma, Refractory Mantle Cell Lymphoma, Refractory Transformed Non-Hodgkin Lymphoma, Recurrent Transformed B-cell Non-Hodgkin Lymphoma, Recurrent Transformed Chronic Lymphocytic Leukemia, Refractory Marginal Zone Lymphoma, Refractory Transformed B-cell Non-Hodgkin Lymphoma, Refractory Transformed Chronic Lymphocytic Leukemia | Recruiting | Fred Hutchinson Cancer Research Center | September 11, 2017 |
| CAR-T | NCT04089215 | CD19-targeted CAR-T Cells for Relapsed and Refractory (R/R) Non-Hodgkins Lymphoma | Lymphoma, Non-Hodgkin, Diffuse Large B Cell Lymphoma, Follicular Lymphoma | Recruiting | Shanghai Ming Ju Biotechnology Co., Ltd. | September 13, 2019 |
| CAR-T | NCT03614858 | CD19/CD22-targeted Chimeric Antigen Receptor Engineered T Cell (CART) in B-Cell Acute Lymphoblastic Leukemia. | Leukemia, B-cell | Recruiting | Shanghai Unicar-Therapy Bio-medicine Technology Co.,Ltd | August 3, 2018 |
| CAR-T | NCT03999697 | A Clinical Research of CD22-Targeted CAR-T in B Cell Malignancies | Diffuse Large B Cell Lymphoma, Follicular Lymphoma, Primary Cutaneous Follicle Centre Lymphoma, Extranodal Marginal Zone Lymphoma of Mucosa-Associated Lymphoid Tissue, Mantle Cell Lymphoma, Plasma Cell Neoplasm, B Cell Lymphoma | Recruiting | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | June 27, 2019 |
| CAR-T | NCT04007029 | Modified Immune Cells (CD19/CD20 CAR-T Cells) in Treating Patients With Recurrent or Refractory B-Cell Lymphoma or Chronic Lymphocytic Leukemia | CD19 Positive, CD20 Positive, Recurrent Chronic Lymphocytic Leukemia, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Follicular Lymphoma, Recurrent Mantle Cell Lymphoma, Recurrent Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma, Recurrent Small Lymphocytic Lymphoma, Refractory Chronic Lymphocytic Leukemia, Refractory Diffuse Large B-Cell Lymphoma, Refractory Follicular Lymphoma, Refractory Mantle Cell Lymphoma, Refractory Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma, Refractory Small Lymphocytic Lymphoma | Not yet recruiting | Jonsson Comprehensive Cancer Center | July 3, 2019 |
| CAR-T | NCT04003649 | IL13Ralpha2-Targeted Chimeric Antigen Receptor (CAR) T Cells With or Without Nivolumab and Ipilimumab in Treating Patients With Recurrent or Refractory Glioblastoma | Recurrent Glioblastoma, Refractory Glioblastoma | Not yet recruiting | City of Hope Medical Center | July 1, 2019 |
| CAR-T | NCT04008251 | Humanized CD19 Chimeric Antigen Receptor (CAR)-Modified T Cell Therapy in Treating Patients With B-cell Malignancies | Acute Lymphoblastic Leukemia, B Cell Lymphoma | Recruiting | Wuhan Sian Medical Technology Co., Ltd | July 4, 2019 |
| CAR-T | NCT02765243 | Anti-GD2 4th Generation CAR-T Cells Targeting Refractory and/or Recurrent Neuroblastoma | Neuroblastoma, Effects of Immunotherapy | Recruiting | Zhujiang Hospital | May 6, 2016 |
| CAR-T | NCT02965092 | CD19 Chimeric Antigen Receptor (CAR)-Modified T Cell Therapy in Treating Patients With B-cell Malignancies | Acute Lymphoblastic Leukemia, B Cell Lymphoma | Recruiting | Wuhan Sian Medical Technology Co., Ltd | November 16, 2016 |
| CAR-T | NCT02349724 | A Clinical Research of CAR-T Cells Targeting CEA Positive Cancer | Lung Cancer, Colorectal Cancer, Gastric Cancer, Breast Cancer, Pancreatic Cancer | Recruiting | Southwest Hospital, China | January 29, 2015 |
| CAR-T | NCT03851146 | A Study of Anti-Lewis Y Chimeric Antigen Receptor-T Cells (LeY-CAR-T) in Patients With Solid Tumours | Advanced Cancer | Recruiting | Peter MacCallum Cancer Centre, Australia | February 22, 2019 |
| CAR-T | NCT03448393 | CD19/CD22 Chimeric Antigen Receptor (CAR) T Cells in Children and Young Adults With Recurrent or Refractory CD19/CD22-expressing B Cell Malignancies | Acute Lymphoid Leukemia, B-Cell Leukemia, Leukemia, Lymphocytic, B Cell, B-Cell Lymphoma, Lymphoma, Non-Hodgkin | Recruiting | National Cancer Institute (NCI) | February 28, 2018 |
| CAR-T | NCT03954106 | A Safety and Efficacy Study of Defibrotide in the Prevention of Chimeric Antigen Receptor-T-cell-associated Neurotoxicity | DLBCL, Neurotoxicity Syndromes | Not yet recruiting | Jazz Pharmaceuticals | May 17, 2019 |
| CAR-T | NCT02830724 | Administering Peripheral Blood Lymphocytes Transduced With a CD70-Binding Chimeric Antigen Receptor to People With CD70 Expressing Cancers | Pancreatic Cancer, Renal Cell Cancer, Breast Cancer, Melanoma, Ovarian Cancer | Recruiting | National Cancer Institute (NCI) | July 13, 2016 |
| CAR-T | NCT02772198 | T-cells Expressing Anti-CD19 CAR in Pediatric and Young Adults With B-cell Malignancies | Acute Lymphoblastic Leukemia, B-precursor, Non-Hodgkin Lymphoma, B-cell | Recruiting | Sheba Medical Center | May 13, 2016 |
| CAR-T | NCT04025216 | A Study of CART-TnMUC1 in Patients With TnMUC1-Positive Advanced Cancers | Non-Small Cell Lung Cancer, Ovarian Cancer, Fallopian Tube Cancer, Triple Negative Breast Cancer, Multiple Myeloma, Pancreatic Ductal Adenocarcinoma | Not yet recruiting | Tmunity Therapeutics | July 18, 2019 |
| CAR-T | NCT02650999 | Phase I/II Study of Pembrolizumab in Patients Failing to Respond to or Relapsing After Anti-CD19 Chimeric Antigen Receptor Modified T Cell Therapy for Relapsed or Refractory CD19+ Lymphomas | CD19+ Diffuse Large B-cell Lymphomas, Follicular Lymphomas, Mantle Cell Lymphomas | Active, not recruiting | Abramson Cancer Center of the University of Pennsylvania | January 8, 2016 |
| CAR-T | NCT03638167 | EGFR806-specific CAR-T Cell Locoregional Immunotherapy for EGFR-positive Recurrent or Refractory Pediatric CNS Tumors | Central Nervous System Tumor, Pediatric, Glioma, Ependymoma, Medulloblastoma, Germ Cell Tumor, Atypical Teratoid/Rhabdoid Tumor, Primitive Neuroectodermal Tumor, Choroid Plexus Carcinoma, Pineoblastoma | Recruiting | Seattle Children's Hospital | August 20, 2018 |
| CAR-T | NCT03126864 | Study of Adoptive Cellular Therapy Using Autologous T Cells Transduced With Lentivirus to Express a CD33 Specific Chimeric Antigen Receptor in Patients With Relapsed or Refractory CD33-Positive Acute Myeloid Leukemia | Hematopoietic/Lymphoid Cancer, Acute Myeloid Leukemia | Active, not recruiting | M.D. Anderson Cancer Center | April 24, 2017 |
| CAR-T | NCT03497819 | Autologous CARTmeso/19 Against Pancreatic Cancer | Pancreatic Cancer | Active, not recruiting | First Affiliated Hospital of Wenzhou Medical University | April 13, 2018 |
| CAR-T | NCT02640209 | Pilot Trial Of Autologous T Cells Engineered To Express Anti-CD19 Chimeric Antigen Receptor (CART19)In Combination With Ibrutinib In Patients With Relapsed Or Refractory CD19+ Chronic Lymphocytic Leukemia (CLL)Or Small Lymphocytic Lymphoma (SLL) | LYMPHOCYTIC LEUKEMIA (CLL) OR SMALL LYMPHOCYTIC LYMPHOMA (SLL) | Active, not recruiting | University of Pennsylvania | December 28, 2015 |
| CAR-T | NCT03502577 | BCMA-Specific CAR T-Cells Combined With a Gamma Secretase Inhibitor (JSMD194) to Treat Relapsed or Persistent Multiple Myeloma | Recurrent Plasma Cell Myeloma, Refractory Plasma Cell Myeloma | Recruiting | Fred Hutchinson Cancer Research Center | April 18, 2018 |
| CAR-T | NCT03829540 | CD4CAR for CD4+ Leukemia and Lymphoma | T-cell Lymphoma, T-cell Leukemia | Recruiting | Stony Brook University | February 4, 2019 |
| CAR-T | NCT02919046 | Study Evaluating the Efficacy and Safety With CAR-T for Relapsed or Refractory Neuroblastoma in Children | Relapsed or Refractory Neuroblastoma | Recruiting | Sinobioway Cell Therapy Co., Ltd. | September 29, 2016 |
| CAR-T | NCT03355859 | Study Evaluating the Safety and Efficacy of JWCAR029 in Adult Subjects With Relapsed and Refractory B-cell Non-Hodgkin Lymphoma | Non Hodgkin Lymphoma | Active, not recruiting | Zhao Weili | November 28, 2017 |
| CAR-T | NCT03344367 | Study Evaluating the Safety and Efficacy of JWCAR029 in Adult Subjects With Relapsed and Refractory B-cell Non-Hodgkin Lymphoma | Non Hodgkin Lymphoma | Active, not recruiting | Peking University | November 17, 2017 |
| CAR-T | NCT01840566 | High Dose Therapy and Autologous Stem Cell Transplantation Followed by Infusion of Chimeric Antigen Receptor (CAR) Modified T-Cells Directed Against CD19+ B-Cells for Relapsed and Refractory Aggressive B Cell Non-Hodgkin Lymphoma | Non-Hodgkin's Lymphoma | Active, not recruiting | Memorial Sloan Kettering Cancer Center | April 25, 2013 |
| CAR-T | NCT02529813 | CD19-Specific T-cells in Treating Patients With Advanced Lymphoid Malignancies | Acute Biphenotypic Leukemia, Acute Lymphoblastic Leukemia, Blasts 5 Percent or More of Bone Marrow Nucleated Cells, CD19 Positive, Minimal Residual Disease, Non-Hodgkin Lymphoma, Small Lymphocytic Lymphoma, Stage III Chronic Lymphocytic Leukemia, Stage IV Chronic Lymphocytic Leukemia | Active, not recruiting | M.D. Anderson Cancer Center | August 20, 2015 |
| CAR-T | NCT03919240 | CAR-T Cell Therapy Targeting to CD19 for R/R ALL | Acute Lymphoblastic Leukemia With Failed Remission | Recruiting | The First Affiliated Hospital of Soochow University | April 18, 2019 |
| CAR-T | NCT03483688 | A PhaseⅠb Study Evaluating Safety and Efficacy of C-CAR011 Treatment in B- NHL Subjects | B-cell Non-Hodgkin Lymphoma | Not yet recruiting | Peking Union Medical College Hospital | March 30, 2018 |
| CAR-T | NCT02915445 | EpCAM CAR-T for Treatment of Nasopharyngeal Carcinoma and Breast Cancer | Malignant Neoplasm of Nasopharynx TNM Staging Distant Metastasis (M), Breast Cancer Recurrent | Recruiting | Sichuan University | September 27, 2016 |
| CAR-T | NCT03373097 | Anti-GD2 CAR-T Cells in Pediatric Patients Affected by High Risk and/or Relapsed/Refractory Neuroblastoma | Neuroblastoma, Neuroblastoma Recurrent | Recruiting | Bambino Gesù Hospital and Research Institute | December 14, 2017 |
| CAR-T | NCT02664363 | EGFRvIII CAR-T Cells for Newly-Diagnosed WHO Grade IV Malignant Glioma | Glioblastoma, Gliosarcoma | Active, not recruiting | Gary Archer Ph.D. | January 27, 2016 |
| CAR-T | NCT03373071 | Anti-CD19 CAR-T Cells in Pediatric Patients Affected by Relapsed/Refractory CD19+ ALL and NHL | CD19-ALL, CD19-LNH | Recruiting | Bambino Gesù Hospital and Research Institute | December 14, 2017 |
| CAR-T | NCT03344705 | Safety and Efficacy Evaluation of IM19 Cells | Hematological Malignancies | Recruiting | Beijing Immunochina Medical Science & Technology Co., Ltd. | November 17, 2017 |
| CAR-T | NCT03873805 | PSCA-CAR T Cells in Treating Patients With Metastatic Castration Resistant Prostate Cancer | Castration Levels of Testosterone, Castration-Resistant Prostate Carcinoma, Metastatic Prostate Carcinoma, PSA Progression, Stage IV Prostate Cancer AJCC v8, Stage IVA Prostate Cancer AJCC v8, Stage IVB Prostate Cancer AJCC v8, PSCA Positive | Recruiting | City of Hope Medical Center | March 13, 2019 |
| CAR-T | NCT03599375 | Immunotherapy With CD19 CART-cells for B Cell Acute Lymphoblastic Leukemia | Leukemia, B-Cell | Not yet recruiting | jiuwei cui | July 25, 2018 |
| CAR-T | NCT01683279 | A Pediatric Trial of Genetically Modified Autologous T Cells Directed Against CD19 for Relapsed CD19+ Acute Lymphoblastic Leukemia | B Cell Leukemia | Active, not recruiting | Seattle Children's Hospital | September 11, 2012 |
| CAR-T | NCT03840317 | Senl_1904A and Senl_1904B Chimeric Antigen Receptor (CAR) T-Cell in the Treatment of r/ r Acute B Lymphocytic Leukemia | Acute Lymphocytic Leukemia | Recruiting | Hebei Senlang Biotechnology Inc., Ltd. | February 15, 2019 |
| CAR-T | NCT03114670 | Donor-derived Anti-CD123-CART Cells for Recurred AML After Allo-HSCT | Adult Acute Myeloid Leukemia | Recruiting | Affiliated Hospital to Academy of Military Medical Sciences | April 14, 2017 |
| CAR-T | NCT03240328 | The Effect of Chimeric Antigen Receptor (CAR)-T Cell Therapy on the Reconstitution of HIV-specific Immune Function | HIV/AIDS | Recruiting | Guangzhou 8th People's Hospital | August 7, 2017 |
| CAR-T | NCT03262298 | Anti-CD22 CAR-T Cell Therapy Targeting B Cell Malignancies | Leukemia, Lymphoma | Recruiting | Affiliated Hospital to Academy of Military Medical Sciences | August 25, 2017 |
| CAR-T | NCT03275493 | Humanized CD19 CAR-T Cells With CRS Suppression Technology for r/r CD19+ Acute Lymphoblastic Leukemia | Acute Lymphoblastic Leukemia, CD19 Positive, Relapse, Refractory | Recruiting | Shanghai Unicar-Therapy Bio-medicine Technology Co.,Ltd | September 7, 2017 |
| CAR-T | NCT02761915 | A Cancer Research UK Trial of Anti-GD2 T-cells (1RG-CART) | Relapsed or Refractory Neuroblastoma | Recruiting | Cancer Research UK | May 4, 2016 |
| CAR-T | NCT03298828 | CD19 CAR and PD-1 Knockout Engineered T Cells for CD19 Positive Malignant B-cell Derived Leukemia and Lymphoma | Acute Lymphoblastic Leukemia, Burkitt Lymphoma | Not yet recruiting | Third Military Medical University | October 2, 2017 |
| CAR-T | NCT04020575 | Autologous huMNC2-CAR44 T Cells for Breast Cancer Targeting Cleaved Form of MUC1 (MUC1*) | Metastatic Breast Cancer | Not yet recruiting | Minerva Biotechnologies Corporation | July 16, 2019 |
| CAR-T | NCT03706547 | Anti-CD19/BCMA Bispecific CAR-T Cell Therapy for R/R MM | Multiple Myeloma in Relapse, Multiple Myeloma Progression | Not yet recruiting | Peng Liu | October 16, 2018 |
| CAR-T | NCT04094766 | Safety and Efficacy of Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy in R/R Acute B Lymphoblastic Leukemia | Refractory B Acute Lymphoblastic Leukemia, Relapse B Acute Lymphoblastic Leukemia | Recruiting | Second Affiliated Hospital of Xi'an Jiaotong University | September 19, 2019 |
| CAR-T | NCT03593109 | Safety and Efficacy Study of Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy in Relapsed or Refractory Lymphoma | Lymphoma, Lymphoma, B-Cell, Lymphoma, Large B-Cell, Diffuse | Recruiting | Second Affiliated Hospital of Xi'an Jiaotong University | July 19, 2018 |
| CAR-T | NCT03710421 | CS1-CAR T Therapy Following Chemotherapy in Treating Patients With Relapsed or Refractory CS1 Positive Multiple Myeloma | Recurrent Plasma Cell Myeloma, Refractory Plasma Cell Myeloma, SLAMF7 Positive | Recruiting | City of Hope Medical Center | October 18, 2018 |
| CAR-T | NCT03896854 | CART-19 T Cell in CD19 Positive Relapsed or Refractory Acute Myeloid Leukemia (AML) | Acute Myeloid Leukemia | Recruiting | Shanghai Unicar-Therapy Bio-medicine Technology Co.,Ltd | April 1, 2019 |
| CAR-T | NCT02799550 | Allogeneic CART-19 for Elderly Relapsed/Refractory CD19+ ALL | Leukemia | Recruiting | The Affiliated Hospital of the Chinese Academy of Military Medical Sciences | June 15, 2016 |
| CAR-T | NCT03854994 | CD19 CAR-T Cell Therapy for Relapsed/Refractory B-cell Lymphoma and B-cell Acute Lymphoblastic Leukemia | B Cell Lymphoma, B-cell Acute Lymphoblastic Leukemia | Recruiting | Yan'an Affiliated Hospital of Kunming Medical University | February 26, 2019 |
| CAR-T | NCT02706405 | JCAR014 and Durvalumab in Treating Patients With Relapsed or Refractory B-cell Non-Hodgkin Lymphoma | BCL2 Gene Rearrangement, BCL6 Gene Rearrangement, CD19 Positive, Diffuse Large B-Cell Lymphoma, Not Otherwise Specified, High-Grade B-Cell Lymphoma With MYC, BCL2, and BCL6 Rearrangements, MYC Gene Rearrangement, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma | Recruiting | Fred Hutchinson Cancer Research Center | March 11, 2016 |
| CAR-T | NCT02842138 | Autologous CD19 CAR-T Cells in Relapsed or Refractory B-cell Lymphoma | B-cell Lymphoma | Active, not recruiting | Peking University | July 22, 2016 |
| CAR-T | NCT03473496 | CAR-T Cells Therapy in Relapsed/Refractory Multiple Myeloma | Relapsed/Refractory Multiple Myeloma(MM) | Recruiting | Zhujiang Hospital | March 22, 2018 |
| CAR-T | NCT02650414 | CD22 Redirected Autologous T Cells for ALL | B Cell Leukemias, B Cell Lymphomas | Recruiting | University of Pennsylvania | January 8, 2016 |
| CAR-T | NCT02431988 | Evaluation of CAR19 T-cells as an Optimal Bridge to Allogeneic Transplantation | Diffuse Large B-Cell Lymphoma | Recruiting | University College, London | May 1, 2015 |
| CAR-T | NCT03721068 | Study of CAR T-Cells Targeting the GD2 With IL-15+iCaspase9 for Relapsed/Refractory Neuroblastoma | Neuroblastoma | Recruiting | UNC Lineberger Comprehensive Cancer Center | October 26, 2018 |
| CAR-T | NCT02537977 | CD19-directed CAR-T Cells Therapy in Relapsed/Refractory B Cell Malignancy | Leukemia, Lymphoma | Recruiting | Shanghai Tongji Hospital, Tongji University School of Medicine | September 2, 2015 |
| CAR-T | NCT04067414 | Exploratory Study of CD19-targeted Chimeric Antigen Receptor T Cells(BZ019) for Relapsed and Refractory B-cell Lymphoma | Diffuse Large B Cell Lymphoma, High-grade B-cell Lymphoma, Transformed Lymphoma, Primary Mediastinal Large B Cell Lymphoma | Recruiting | Institute of Hematology & Blood Diseases Hospital | August 26, 2019 |
| CAR-T | NCT03602157 | Study of CAR-T Cells Expressing CD30 and CCR4 for r/r CD30+ HL and NHL | Lymphoma, Lymphoma, Non-Hodgkin, Immune System Diseases, Immunoproliferative Disorders, Lymphatic Diseases, Lymphoproliferative Disorders, Neoplasms, Cutaneous Lymphoma, Cutaneous Anaplastic Large Cell Lymphoma, Mycosis Fungoides, Sezary Syndrome, Lymphomatoid Papulosis | Recruiting | UNC Lineberger Comprehensive Cancer Center | July 26, 2018 |
| CAR-T | NCT03283631 | Intracerebral EGFR-vIII CAR-T Cells for Recurrent GBM | Recurrent Glioblastoma, Recurrent Gliosarcoma | Recruiting | Gary Archer Ph.D. | September 14, 2017 |
| CAR-T | NCT03103971 | huJCAR014 CAR-T Cells in Treating Adult Patients With Relapsed or Refractory B-Cell Non-Hodgkin Lymphoma or Acute Lymphoblastic Leukemia | Adult B Acute Lymphoblastic Leukemia, BCL2 Gene Rearrangement, BCL6 Gene Rearrangement, CD19 Positive, Diffuse Large B-Cell Lymphoma, Not Otherwise Specified, MYC Gene Rearrangement, Recurrent Adult Acute Lymphoblastic Leukemia, Recurrent B-Cell Non-Hodgkin Lymphoma, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma, Refractory Adult Acute Lymphoblastic Leukemia, Refractory B-Cell Non-Hodgkin Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Primary Mediastinal (Thymic) Large B-Cell Cell Lymphoma, Recurrent Transformed Non-Hodgkin Lymphoma | Recruiting | Fred Hutchinson Cancer Research Center | April 7, 2017 |
| CAR-T | NCT03190278 | Study Evaluating Safety and Efficacy of UCART123 in Patients With Acute Myeloid Leukemia | Acute Myeloid Leukemia | Recruiting | Cellectis S.A. | June 16, 2017 |
| CAR-T | NCT03473457 | CAR-T Cells Therapy in Relapsed/Refractory Acute Myeloid Leukemia | Relapsed/Refractory Acute Myeloid Leukemia(AML) | Recruiting | Zhujiang Hospital | March 22, 2018 |
| CAR-T | NCT04083495 | CD30 CAR for Relapsed/Refractory CD30+ T Cell Lymphoma | Peripheral T Cell Lymphoma | Not yet recruiting | UNC Lineberger Comprehensive Cancer Center | September 10, 2019 |
| CAR-T | NCT02311621 | Engineered Neuroblastoma Cellular Immunotherapy (ENCIT)-01 | Neuroblastoma, Ganglioneuroblastoma | Recruiting | Seattle Children's Hospital | December 8, 2014 |
| CAR-T | NCT02851589 | Study Evaluating the Efficacy and Safety of PCAR-019 in CD19 Positive Relapsed or Refractory Leukemia and Lymphoma | Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Follicular Lymphoma, Mantle Cell Lymphoma, B-cell Prolymphocytic Leukemia, Diffuse Large Cell Lymphoma | Recruiting | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | August 1, 2016 |
| CAR-T | NCT03617198 | CD4 CAR+ ZFN-modified T Cells in HIV Therapy | Hiv | Recruiting | University of Pennsylvania | August 6, 2018 |
| CAR-T | NCT02690545 | Study of CD30 CAR for Relapsed/Refractory CD30+ HL and CD30+ NHL | Lymphoma, Lymphoma, Non-Hodgkin, Immune System Diseases, Immunoproliferative Disorders, Lymphatic Diseases, Lymphoproliferative Disorders, Neoplasms, Neoplasms by Histologic Type | Recruiting | UNC Lineberger Comprehensive Cancer Center | February 24, 2016 |
| CAR-T | NCT02050347 | Activated T Lymphocytes Expressing CARs, Relapsed CD19+ Malignancies Post-Allo HSCT(CARPASCIO) | Non-Hodgkin's Lymphoma, B-Cell ALL, B-Cell CLL | Recruiting | Baylor College of Medicine | January 30, 2014 |
| CAR-T | NCT02672501 | A Study to Assess CD19-targeted Immunotherapy T Cells in Patients With Relapsed or Refractory CD19+ B Cell Leukemia | Leukemia, B-Cell | Recruiting | Shanghai GeneChem Co., Ltd. | February 3, 2016 |
| CAR-T | NCT03081910 | Autologous T-Cells Expressing a Second Generation CAR for Treatment of T-Cell Malignancies Expressing CD5 Antigen | T-cell Acute Lymphoblastic Lymphoma, T-non-Hodgkin Lymphoma, T-cell Acute Lymphoblastic Leukemia | Recruiting | Baylor College of Medicine | March 16, 2017 |
| CAR-T | NCT02663297 | Administration of T Lymphocytes for Prevention of Relapse of Lymphomas | Hodgkin Disease, Lymphoma, Lymphoma, Non-Hodgkin, Immune System Diseases, Immunoproliferative Disorders, Lymphatic Diseases, Lymphoproliferative Disorders, Neoplasms, Neoplasms by Histologic Type | Recruiting | UNC Lineberger Comprehensive Cancer Center | January 26, 2016 |
| CAR-T | NCT02933775 | CD19-redirected Autologous Cells (CAR-CD19 T Cells) | CD19 Positive Malignant B-cell Leukemia and Lymphoma | Not yet recruiting | RenJi Hospital | October 14, 2016 |
| CAR-T | NCT01953900 | iC9-GD2-CAR-VZV-CTLs/Refractory or Metastatic GD2-positive Sarcoma and Neuroblastoma | Osteosarcoma, Neuroblastoma | Recruiting | Baylor College of Medicine | October 1, 2013 |
| CAR-T | NCT03281551 | Efficacy and Safety of PZ01 Treatment in Patients With r/r CD19+ B-cell Acute Lymphoblastic Leukemia/B Cell Lymphoma | B-cell Acute Lymphoblastic Leukemia, B-cell Lymphoma | Recruiting | Pinze Lifetechnology Co. Ltd. | September 13, 2017 |
| CAR-T | NCT03159819 | Clinical Study of CAR-CLD18 T Cells in Patients With Advanced Gastric Adenocarcinoma and Pancreatic Adenocarcinoma | Advanced Gastric Adenocarcinoma, Pancreatic Adenocarcinoma | Recruiting | Changhai Hospital | May 19, 2017 |
| CAR-T | NCT03716856 | Clinical Study of CAR-BCMA T in Patients With Refractory or Relapsed Multiple Myeloma | Refractory or Relapsed Multiple Myeloma | Recruiting | First Affiliated Hospital of Zhejiang University | October 23, 2018 |
| CAR-T | NCT03380039 | Clinical Study of CAR-BCMA T Cells in Patients With Refractory or Relapsed Multiple Myeloma | Refractory or Relapsed Multiple Myeloma | Recruiting | Xinhua Hospital, Shanghai Jiao Tong University School of Medicine | December 20, 2017 |
| CAR-T | NCT01316146 | Administration of T Lymphocytes for Hodgkin's Lymphoma and Non-Hodgkin's Lymphoma (CART CD30) | Non-Hodgkin's Lymphoma, Hodgkin's Lymphoma | Active, not recruiting | UNC Lineberger Comprehensive Cancer Center | March 16, 2011 |
| CAR-T | NCT03685786 | CART19 Cells Treatment of MRD of B Cell Malignancies and Then Auto-HSCT | Leukemia, Lymphocytic, Acute, B-Cell, Leukemia, Lymphocytic, Chronic, B-Cell, Lymphoma, B-Cell | Recruiting | Shenzhen Second People's Hospital | September 26, 2018 |
| CAR-T | NCT03642626 | MT2017-45: CAR-T Cell Therapy for Heme Malignancies | Acute Lymphoblastic Leukemia, Large B-cell Lymphoma | Recruiting | Masonic Cancer Center, University of Minnesota | August 22, 2018 |
| CAR-T | NCT02081937 | CART-19 Immunotherapy in Mantle Cell Lymphoma | Hematopoietic/Lymphoid Cancer, Non-hodgkin Lymphoma,B Cell, Mantle Cell Lymphoma | Recruiting | Chinese PLA General Hospital | March 7, 2014 |
| CAR-T | NCT04088890 | Autologous CD22 CAR-T Cells in Adults w/ Recurrent or Refractory B Cell Malignancies | B-ALL, B-cell Non Hodgkin Lymphoma, DLBCL | Recruiting | Stanford University | September 13, 2019 |
| CAR-T | NCT04088864 | CD22-CAR T Cells in Children and Young Adults With B Cell Malignancies | B Cell Lymphoma, Acute Lymphoblastic Leukemia, Pediatric, Lymphoma | Not yet recruiting | Stanford University | September 13, 2019 |
| CAR-T | NCT03093168 | BCMA Chimeric Antigen Receptor Expressing T Cells in Multiple Myeloma | Multiple Myeloma | Recruiting | The Second Affiliated Hospital of Henan University of Traditional Chinese Medicine | March 28, 2017 |
| CAR-T | NCT03943472 | BCMA Chimeric Antigen Receptor Expressing T Cells Therapy for Relapsed/Refractory Multiple Myeloma | Multiple Myeloma | Not yet recruiting | Hrain Biotechnology Co., Ltd. | May 9, 2019 |
| CAR-T | NCT03598179 | XLCART001 Treatment in Relapsed/Refractory/High-risk B-cell Malignancy Subjects | Lymphoma, B-Cell, Leukemia, B-cell | Active, not recruiting | The First Affiliated Hospital with Nanjing Medical University | July 25, 2018 |
| CAR-T | NCT03070327 | BCMA Targeted CAR-T Cells With or Without Lenalidomide for the Treatment of Multiple Myeloma | Multiple Myeloma | Recruiting | Memorial Sloan Kettering Cancer Center | March 3, 2017 |
| CAR-T | NCT03244306 | A Phase 1 Study of CD22-CAR TCell Immunotherapy for CD22+ Leukemia and Lymphoma | Leukemia | Active, not recruiting | Seattle Children's Hospital | August 9, 2017 |
| CAR-T | NCT04007978 | Anti-CD22 Chimeric Antigen Receptor (CAR)-Modified T Cell Therapy for Relapsed Refractory B-cell Malignancies | B Cell Lymphoma, Acute Lymphoblastic Leukemia | Recruiting | Wuhan Union Hospital, China | July 4, 2019 |
| CAR-T | NCT04034446 | CD19-CD22 Chimeric Antigen Receptor T (CAR-T) Cell for Treatment of B Cell Acute Lymphoblastic Leukemia (B-ALL) | Relapsed or Refractory B Cell Acute Lymphoblastic Leukemia | Not yet recruiting | Institute of Hematology & Blood Diseases Hospital | July 26, 2019 |
| CAR-T | NCT03585517 | Safety and Efficacy Evaluation of IM23 CAR-T Cells (IM23CAR-T) | AML | Recruiting | Beijing Immunochina Medical Science & Technology Co., Ltd. | July 13, 2018 |
| CAR-T | NCT03915184 | Clinical Trial to Evaluate CT053 in Patients With Relapsed and/or Refractory Multiple Myeloma | Multiple Myeloma | Not yet recruiting | Carsgen Therapeutics, Ltd. | April 15, 2019 |
| CAR-T | NCT03692429 | alloSHRINK - Standard cHemotherapy Regimen and Immunotherapy With Allogeneic NKG2D-based CYAD-101 Chimeric Antigen Receptor T-cells | Colorectal Cancer | Recruiting | Celyad (formerly named Cardio3 BioSciences) | October 2, 2018 |
| CAR-T | NCT03430011 | Study Evaluating the Safety and Efficacy of JCARH125 in Subjects With Relapsed and/or Refractory Multiple Myeloma | Multiple Myeloma | Recruiting | Juno Therapeutics, Inc. | February 12, 2018 |
| CAR-T | NCT01865617 | Laboratory Treated T Cells in Treating Patients With Relapsed or Refractory Chronic Lymphocytic Leukemia, Non-Hodgkin Lymphoma, or Acute Lymphoblastic Leukemia | CD19-Positive Neoplastic Cells Present, Recurrent Adult Acute Lymphoblastic Leukemia, Recurrent Chronic Lymphocytic Leukemia, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Mantle Cell Lymphoma, Recurrent Non-Hodgkin Lymphoma, Recurrent Small Lymphocytic Lymphoma, Refractory Acute Lymphoblastic Leukemia, Refractory Chronic Lymphocytic Leukemia, Refractory Diffuse Large B-Cell Lymphoma, Refractory Mantle Cell Lymphoma, Refractory Non-Hodgkin Lymphoma, Refractory Small Lymphocytic Lymphoma | Active, not recruiting | Fred Hutchinson Cancer Research Center | May 31, 2013 |
| CAR-T | NCT02631044 | Study Evaluating the Safety and Pharmacokinetics of JCAR017 in B-cell Non-Hodgkin Lymphoma (TRANSCEND-NHL-001) | Non-Hodgkin Lymphoma, Diffuse Large B Cell Lymphoma, Follicular Lymphoma, Mantle-cell Lymphoma, Primary Mediastinal B-cell Lymphoma | Recruiting | Juno Therapeutics, Inc. | December 15, 2015 |
| CAR-T | NCT03720496 | Treatment of Refractory/Relapsed Non-Hodgkin Lymphoma With CD19-TriCAR-T Cell Therapy | Non-hodgkin Lymphoma,B Cell | Recruiting | Timmune Biotech Inc. | October 25, 2018 |
| CAR-T | NCT03497533 | Treatment of Refractory/Relapsed Non-Hodgkin Lymphoma With TriCAR-T_CD19 | Non-hodgkin Lymphoma,B Cell | Recruiting | Timmune Biotech Inc. | April 13, 2018 |
| CAR-T | NCT03186118 | Pilot Study of T-APCs Following CAR-T Cell Immunotherapy for CD19+ Leukemia | CD 19+ Acute Leukemia | Recruiting | Seattle Children's Hospital | June 14, 2017 |
| CAR-T | NCT04011293 | A Clinical Study of Anti-CD19 Chimeric Antigen Receptor T-Cell Injection (CNCT19) in the Treatment of Cluster of Differentiation 19 (CD19) Positive Relapsed or Refractory B Cell Malignancies | Relapsed or Refractory Hematological Malignancies | Recruiting | Shandong University | July 8, 2019 |
| CAR-T | NCT02792114 | T-Cell Therapy for Advanced Breast Cancer | Breast Cancer, Metastatic HER2-negative Breast | Recruiting | Memorial Sloan Kettering Cancer Center | June 7, 2016 |
| CAR-T | NCT03056339 | Umbilical & Cord Blood (CB) Derived CAR-Engineered NK Cells for B Lymphoid Malignancies | B-Lymphoid Malignancies, Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Non-hodgkin Lymphoma | Recruiting | M.D. Anderson Cancer Center | February 17, 2017 |
| CAR-T | NCT04077866 | B7-H3 CAR-T for Recurrent or Refractory Glioblastoma | Recurrent Glioblastoma, Refractory Glioblastoma | Not yet recruiting | Second Affiliated Hospital, School of Medicine, Zhejiang University | September 4, 2019 |
| CAR-T | NCT03060356 | Autologous T Cells Expressing MET scFv CAR (RNA CART-cMET) | Malignant Melanoma, Breast Cancer | Active, not recruiting | University of Pennsylvania | February 23, 2017 |
| CAR-T | NCT02546739 | Immunotherapy With CD19 CAR T-cells for B-Cell Lymphoma, ALL and CLL | Leukemia, Lymphoma | Not yet recruiting | Beijing Doing Biomedical Co., Ltd. | September 11, 2015 |
| CAR-T | NCT02656147 | Immunotherapy With CD19 CAR γδT-cells for B-Cell Lymphoma, ALL and CLL | Leukemia, Lymphoma | Not yet recruiting | Beijing Doing Biomedical Co., Ltd. | January 14, 2016 |
| CAR-T | NCT02729493 | Study Evaluating the Efficacy and Safety With CAR-T for Liver Cancer | Liver Neoplasms | Recruiting | Sinobioway Cell Therapy Co., Ltd. | April 6, 2016 |
| CAR-T | NCT02725125 | Study Evaluating the Efficacy and Safety With CAR-T for Stomach Cancer | Stomach Neoplasms | Recruiting | Sinobioway Cell Therapy Co., Ltd. | March 31, 2016 |
| CAR-T | NCT02735291 | Study Evaluating the Efficacy and Safety With CAR-T for Recurrent or Refractory Acute Non T Lymphocyte Leukemia | Leukemia | Recruiting | Sinobioway Cell Therapy Co., Ltd. | April 12, 2016 |
| CAR-T | NCT01318317 | Genetically Engineered Lymphocyte Therapy After Peripheral Blood Stem Cell Transplant in Treating Patients With High-Risk, Intermediate-Grade, B-cell Non-Hodgkin Lymphoma | Recurrent Adult Diffuse Large Cell Lymphoma, Recurrent Adult Diffuse Mixed Cell Lymphoma, Recurrent Adult Diffuse Small Cleaved Cell Lymphoma, Recurrent Grade 3 Follicular Lymphoma, Recurrent Mantle Cell Lymphoma | Active, not recruiting | City of Hope Medical Center | March 18, 2011 |
| CAR-T | NCT02893189 | CAR19 Donor Lymphocytes for Relapsed CD19+ Malignancies Following Allogeneic Transplantation | CD19+ Malignancies: Relapse Post-allogeneic Transplant | Recruiting | University College, London | September 8, 2016 |
| CAR-T | NCT04026737 | Cardiovascular Effects of CAR-T Cell Therapy | Leukemia, Lymphoma, Cardiotoxicity, Risk Factor, Cardiovascular, Immunotherapy | Recruiting | Abramson Cancer Center of the University of Pennsylvania | July 19, 2019 |
| CAR-T | NCT03356795 | Intervention of CAR-T Against Cervical Cancer | Cervical Cancer | Recruiting | Shenzhen Geno-Immune Medical Institute | November 29, 2017 |
| CAR-T | NCT03361748 | Efficacy and Safety Study of bb2121 in Subjects With Relapsed and Refractory Multiple Myeloma (KarMMa) | Multiple Myeloma | Recruiting | Celgene | December 5, 2017 |
| CAR-T | NCT03768310 | CD19.CAR-multiVSTs for Patients With CD19+ B-ALL or NHL Undergoing Related Allogeneic HSCT (CARMA) | Non-hodgkin Lymphoma, Acute Lymphoblastic Leukemia | Not yet recruiting | Baylor College of Medicine | December 7, 2018 |
| CAR-T | NCT03323944 | CAR-T Cell Immunotherapy for Pancreatic Cancer | Pancreatic Cancer, Cancer of the Pancreas | Recruiting | University of Pennsylvania | October 27, 2017 |
| CAR-T | NCT03054298 | CAR-T Cells in Mesothelin Expressing Cancers | Lung Adenocarcinoma, Ovarian Cancer, Peritoneal Carcinoma, Fallopian Tube Cancer, Mesotheliomas Pleural, Mesothelioma Peritoneum | Recruiting | University of Pennsylvania | February 15, 2017 |
| CAR-T | NCT03196830 | CAR-T for R/R B-NHL | Relapsed Non Hodgkin Lymphoma, Refractory Non-Hodgkin Lymphoma, CAR - T CD19/CD20/CD22/CD30 | Recruiting | The First Affiliated Hospital of Soochow University | June 23, 2017 |
| CAR-T | NCT03638193 | Study of Autologous T-cells in Patients With Metastatic Pancreatic Cancer | Pancreatic Cancer | Recruiting | Shenzhen BinDeBio Ltd. | August 20, 2018 |
| CAR-T | NCT03207178 | Sequential Infusion of Anti-CD19 and Anti-CD20 CAR-T Cells Against Relapsed and Refractory B-cell Lymphoma | Recurrent or Refractory B Cell Malignancy | Recruiting | Shanghai Longyao Biotechnology Inc., Ltd. | July 2, 2017 |
| CAR-T | NCT01853631 | Activated T-Cells Expressing 2nd or 3rd Generation CD19-Specific CAR, Advanced B-Cell NHL, ALL, and CLL (SAGAN) | Non-Hodgkin Lymphoma, Chronic Lymphocytic Leukemia, Acute Lymphocytic Leukemia | Recruiting | Baylor College of Medicine | May 15, 2013 |
| CAR-T | NCT03090659 | LCAR-B38M-02 Cells in Treating Relapsed/Refractory (R/R) Multiple Myeloma | Refractory or Relapsed Multiple Myeloma | Enrolling by invitation | Nanjing Legend Biotech Co. | March 27, 2017 |
| CAR-T | NCT03815383 | A Study of BCMA-directed CAR-T Cells Treatment in Subjects With r/r Multiple Myeloma | Relapsed or Refractory Multiple Myeloma | Recruiting | The First Affiliated Hospital with Nanjing Medical University | January 24, 2019 |
| CAR-T | NCT03751293 | A Study of BCMA-directed CAR-T Cells Treatment in Subjects With r/r Multiple Myeloma | Relapsed or Refractory Multiple Myeloma | Recruiting | Hebei Yanda Ludaopei Hospital | November 22, 2018 |
| CAR-T | NCT03993743 | A Study of CD147-targeted CAR-T by Hepatic Artery Infusions for Very Advanced Hepatocellular Carcinoma | Advanced Hepatocellular Carcinoma | Recruiting | Xijing Hospital | June 21, 2019 |
| CAR-T | NCT03792633 | Study of huCART19 for Very High-Risk (VHR) Subsets of Pediatric B-ALL | Acute Lymphoid Leukemia | Recruiting | University of Pennsylvania | January 3, 2019 |
| CAR-T | NCT04045847 | CD147-CART Cells in Patients With Recurrent Malignant Glioma. | Recurrent Glioblastoma, CD147 Positive | Active, not recruiting | Xijing Hospital | August 6, 2019 |
| CAR-T | NCT03960060 | A Study of CCT301-59 CAR-T Therapy in Adult Subjects With Recurrent or Refractory Solid Tumors | Solid Tumor, Soft Tissue Sarcoma, Gastric Cancer, Pancreatic Cancer, Bladder Cancer | Recruiting | Shanghai Sinobioway Sunterra Biotech | May 22, 2019 |
| CAR-T | NCT03672305 | Clinical Study on the Efficacy and Safety of c-Met/PD-L1 CAR-T Cell Injection in the Treatment of HCC | Primary Hepatocellular Carcinoma | Not yet recruiting | The Second Hospital of Nanjing Medical University | September 14, 2018 |
| CAR-T | NCT03767725 | Anti-BCMA or/and Anti-CD19 CAR-T Cells Treatment of Relapsed Multiple Myeloma | Multiple Myeloma in Relapse | Recruiting | Shenzhen Second People's Hospital | December 7, 2018 |
| CAR-T | NCT03299738 | A Study Evaluating Safety and Efficacy of C-CAR011 in Subjects With B-NHL | Refractory or Relapsed B-cell Non-Hodgkin Lymphoma | Not yet recruiting | Cellular Biomedicine Group Ltd. | October 3, 2017 |
| CAR-T | NCT03752541 | Efficacy and Safety Evaluation of BCMA-UCART | Multiple Myeloma | Recruiting | Shanghai Bioray Laboratory Inc. | November 26, 2018 |
| CAR-T | NCT00881920 | Kappa-CD28 T Lymphocytes, Chronic Lymphocytic Leukemia, B-cell Lymphoma or Multiple Myeloma, CHARKALL | Lymphoma, Myeloma, Leukemia | Recruiting | Baylor College of Medicine | April 15, 2009 |
| CAR-T | NCT03085173 | A Trial of "Armored" CAR-T Cells Targeting CD19 For Patients With Relapsed CD19+ Hematologic Malignancies | Chronic Lymphocytic Leukemia (CLL), Relapsed, Refractory | Recruiting | Memorial Sloan Kettering Cancer Center | March 21, 2017 |
| CAR-T | NCT02744287 | Use of Ligand-Inducible Autologous T Cells Engineered to Target PSCA on Tumor Cells in Selected Advanced Solid Tumors | Pancreatic Adenocarcinoma, Gastric Adenocarcinoma, Prostate Adenocarcinoma | Recruiting | Bellicum Pharmaceuticals | April 20, 2016 |
| CAR-T | NCT03229876 | Safety and Efficacy Evaluation of CD19-UCART | Acute Lymphoblastic Leukemia (ALL), Non Hodgkin Lymphoma (NHL) | Recruiting | Shanghai Bioray Laboratory Inc. | July 26, 2017 |
| CAR-T | NCT02924753 | The Safety and Efficacy of CART-19 Cells in B-cell Acute Lymphoblastic Leukemia (B-ALL). | B-cell Acute Lymphoblastic Leukemia | Recruiting | Henan Cancer Hospital | October 5, 2016 |
| CAR-T | NCT03110640 | Anti-CD19 CAR-T Infusion Combined With Allogeneic Stem Cell Transplantation for B-cell Leukemia/Lymphoma | B-cell Adult Acute Lymphoblastic Leukemia, B-cell Chronic Lymphocytic Leukemia, Adult Acute Lymphoblastic Leukemia in Remission, Hematopoietic/Lymphoid Cancer, Refractory Chronic Lymphocytic Leukemia | Not yet recruiting | First Affiliated Hospital of Wenzhou Medical University | April 12, 2017 |
| CAR-T | NCT02685670 | Competitive Transfer of αCD19-TCRz-CD28 and αCD19-TCRz-CD137 CAR-T Cells for B-cell Leukemia/Lymphoma | Hematopoietic/Lymphoid Cancer, Adult Acute Lymphoblastic Leukemia in Remission, B-cell Adult Acute Lymphoblastic Leukemia, B-cell Chronic Lymphocytic Leukemia, Recurrent Adult Diffuse Large Cell Lymphoma, Recurrent Grade 1 Follicular Lymphoma, Recurrent Grade 2 Follicular Lymphoma, Recurrent Grade 3 Follicular Lymphoma, Recurrent Mantle Cell Lymphoma, Refractory Chronic Lymphocytic Leukemia, Stage III Adult Diffuse Large Cell Lymphoma, Stage III Chronic Lymphocytic Leukemia, Stage III Grade 1 Follicular Lymphoma, Stage III Grade 2 Follicular Lymphoma, Stage III Grade 3 Follicular Lymphoma, Stage III Mantle Cell Lymphoma, Stage IV Adult Diffuse Large Cell Lymphoma, Stage IV Chronic Lymphocytic Leukemia, Stage IV Grade 1 Follicular Lymphoma, Stage IV Grade 2 Follicular Lymphoma, Stage IV Grade 3 Follicular Lymphoma, Stage IV Mantle Cell Lymphoma | Recruiting | The Second Affiliated Hospital of Henan University of Traditional Chinese Medicine | February 19, 2016 |
| CAR-T | NCT02903810 | Combination Transfer of αCD19-TCRz-41BB and αCD22-TCRz-41BB CAR-T Cells for B-cell Hematologic Malignancy | Hematopoietic/Lymphoid Cancer | Recruiting | Xuzhou Medical University | September 16, 2016 |
| CAR-T | NCT02735083 | A Study to Evaluate the Long-term Safety of Patients With Advanced Lymphoid Malignancies Who Have Been Previously Administered With UCART19/ALLO-501 | Advanced Lymphoid Malignancies | Enrolling by invitation | Institut de Recherches Internationales Servier | April 12, 2016 |
| CAR-T | NCT03338972 | Immunotherapy With BCMA CAR-T Cells in Treating Patients With BCMA Positive Relapsed or Refractory Multiple Myeloma | Recurrent Plasma Cell Myeloma, Refractory Plasma Cell Myeloma, TNFRSF17 Positive | Recruiting | Fred Hutchinson Cancer Research Center | November 9, 2017 |
| CAR-T | NCT02992210 | Study on GD2 Positive Solid Tumors by 4SCAR-GD2 | Solid Tumor | Recruiting | Shenzhen Geno-Immune Medical Institute | December 14, 2016 |
| CAR-T | NCT03366324 | Anti-CD19 CAR-T Therapy Combine With HSCT to Treat MRD+ B-cell Malignancies | Acute Lymphoblastic Leukemia, B Cell Lymphoma | Recruiting | Wuhan Sian Medical Technology Co., Ltd | December 8, 2017 |
| CAR-T | NCT03013712 | A Clinical Research of CAR-T Cells Targeting EpCAM Positive Cancer | Colon Cancer, Esophageal Carcinoma, Pancreatic Cancer, Prostate Cancer, Gastric Cancer, Hepatic Carcinoma | Recruiting | First Affiliated Hospital of Chengdu Medical College | January 6, 2017 |
| CAR-T | NCT03366350 | Anti-CD19 CAR-T Therapy Bridging to HSCT for CD19+ B-Cell Malignancies | Acute Lymphoblastic Leukemia, B Cell Lymphoma | Recruiting | Wuhan Sian Medical Technology Co., Ltd | December 8, 2017 |
| CAR-T | NCT03765177 | CLIC-1901 for the Treatment of Patients With Relapsed/Refractory CD19 Positive Hematologic Malignancies | Acute Lymphoblastic Leukemia, Non-Hodgkin's Lymphoma | Not yet recruiting | Ottawa Hospital Research Institute | December 5, 2018 |
| CAR-T | NCT03060343 | Zeushield Cytotoxic T Lymphocytes (Z-CTLs) for Relapsed or Refractory Non Small Cell Lung Cancer (NSCLC) | Non-small Cell Lung Cancer | Recruiting | Yu Fenglei | February 23, 2017 |
| CAR-T | NCT03393936 | Safety and Efficacy of CCT301 CAR-T in Adult Subjects With Recurrent or Refractory Stage IV Renal Cell Carcinoma | Renal Cell Carcinoma | Recruiting | Shanghai Sinobioway Sunterra Biotech | January 9, 2018 |
| CAR-T | NCT03620058 | CART22 Alone or in Combination With huCART19 for ALL | Chemotherapy Resistant Acute Lymphoblastic Leukemia, Refractory Acute Lymphoblastic Leukemia | Recruiting | University of Pennsylvania | August 8, 2018 |
| CAR-T | NCT02159495 | Genetically Modified T-cell Immunotherapy in Treating Patients With Relapsed/Refractory Acute Myeloid Leukemia and Persistent/Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm | Adult Acute Myeloid Leukemia in Remission, Acute Biphenotypic Leukemia, Early Relapse of Acute Myeloid Leukemia, Late Relapse of Acute Myeloid Leukemia, Recurrent Adult Acute Myeloid Leukemia, Secondary Acute Myeloid Leukemia, Blastic Plasmacytoid Dendritic Cell Neoplasm, Acute Myeloid Leukemia, Adult Acute Lymphoblastic Leukemia, Interleukin-3 Receptor Subunit Alpha Positive, Minimal Residual Disease, Refractory Acute Myeloid Leukemia, Untreated Adult Acute Myeloid Leukemia | Recruiting | City of Hope Medical Center | June 10, 2014 |
| CAR-T | NCT02746952 | Dose Escalation Study of UCART19 in Adult Patients With Relapsed / Refractory B-cell Acute Lymphoblastic Leukaemia | B-cell Acute Lymphoblastic Leukemia | Recruiting | Institut de Recherches Internationales Servier | April 21, 2016 |
| CAR-T | NCT02315612 | Anti-CD22 Chimeric Receptor T Cells in Pediatric and Young Adults With Recurrent or Refractory CD22-expressing B Cell Malignancies | Follicular Lymphoma, ALL, NHL, Large Cell Lymphoma | Recruiting | National Cancer Institute (NCI) | December 12, 2014 |
| CAR-T | NCT03635632 | C7R-GD2.CART Cells for Patients With Relapsed or Refractory Neuroblastoma (GAIL-N) | Relapsed Neuroblastoma, Refractory Neuroblastoma | Recruiting | Baylor College of Medicine | August 17, 2018 |
| CAR-T | NCT03579927 | CAR.CD19-CD28-zeta-2A-iCasp9-IL15-Transduced Cord Blood NK Cells, High-Dose Chemotherapy, and Stem Cell Transplant in Treating Participants With B-cell Lymphoma | CD19 Positive, Mantle Cell Lymphoma, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Follicular Lymphoma, Refractory B-Cell Non-Hodgkin Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Follicular Lymphoma | Not yet recruiting | M.D. Anderson Cancer Center | July 9, 2018 |
| CAR-T | NCT03155191 | Study of TBI-1501 for Relapsed or Refractory Acute Lymphoblastic Leukemia | Lymphoblastic Leukemia, Acute Adult | Recruiting | Takara Bio Inc. | May 16, 2017 |
| CAR-T | NCT01818323 | Phase I Trial: T4 Immunotherapy of Head and Neck Cancer | Head and Neck Cancer | Recruiting | King's College London | March 26, 2013 |
| CAR-T | NCT02208362 | Genetically Modified T-cells in Treating Patients With Recurrent or Refractory Malignant Glioma | Malignant Glioma, Refractory Brain Neoplasm, Recurrent Brain Neoplasm, Glioblastoma | Recruiting | City of Hope Medical Center | August 5, 2014 |
| CAR-T | NCT03271515 | Immunotherapy With Bispecific CAR-T Cells for B-Cell Lymphoma, ALL and CLL | Leukemia, Lymphoma | Not yet recruiting | Beijing Doing Biomedical Co., Ltd. | September 5, 2017 |
| CAR-T | NCT02963038 | CAR-T Cells for Refractory B Cell Malignancy | B-Cell Leukemia, B-Cell Lymphoma | Recruiting | Hebei Senlang Biotechnology Inc., Ltd. | November 15, 2016 |
| CAR-T | NCT03573700 | Evaluation of CD19-Specific CAR Engineered Autologous T-Cells for Treatment of Relapsed/Refractory CD19+ Acute Lymphoblastic Leukemia | Acute Lymphoblastic Leukemia, in Relapse, Acute Lymphoblastic Leukemia, Refractory | Recruiting | St. Jude Children's Research Hospital | June 29, 2018 |
| CAR-T | NCT03084380 | Anti-GPC3 CAR-T for Treating GPC3-positive Advanced Hepatocellular Carcinoma (HCC) | Hepatocellular Carcinoma | Not yet recruiting | Xinqiao Hospital of Chongqing | March 20, 2017 |
| CAR-T | NCT02028455 | A Pediatric and Young Adult Trial of Genetically Modified T Cells Directed Against CD19 for Relapsed/Refractory CD19+ Leukemia | CD19+ Acute Leukemia | Recruiting | Seattle Children's Hospital | January 7, 2014 |
| CAR-T | NCT03068416 | CD19-targeting, 3rd Generation CAR-T Cells for Refractory B Cells Malignancy | B-cell Leukemia, B-Cell Lymphoma | Recruiting | Uppsala University | March 1, 2017 |
| CAR-T | NCT03064269 | CAR-T Therapy for Central Nervous System B-cell Acute Lymphocytic Leukemia | B-cell Acute Lymphocytic Leukemia | Recruiting | Shanghai Unicar-Therapy Bio-medicine Technology Co.,Ltd | February 27, 2017 |
| CAR-T | NCT03142646 | Safety and Efficacy Evaluation of IM19 CAR-T Cells | Leukemia | Recruiting | Beijing Immunochina Medical Science & Technology Co., Ltd. | May 5, 2017 |
| CAR-T | NCT03814447 | The Fourth Generation CART-cell Therapy for Refractory-Relapsed Ovarian Cancer | Ovarian Cancer | Recruiting | Shanghai 6th People's Hospital | January 24, 2019 |
| CAR-T | NCT03879382 | Anti-CD19/BCMA Bispecific CAR-T Cell Therapy for R/R POMES | POMES Syndrome, Relapsed and Refractory POMES Syndrome | Recruiting | Hrain Biotechnology Co., Ltd. | March 18, 2019 |
| CAR-T | NCT02958384 | A Clinical Research of LeY-Targeted CAR-T in Myeloid Malignancies | Myeloid Malignancies | Recruiting | Southwest Hospital, China | November 8, 2016 |
| CAR-T | NCT02958397 | A Clinical Research of CD33-Targeted CAR-T in Myeloid Malignancies | Myeloid Malignancies | Recruiting | Southwest Hospital, China | November 8, 2016 |
| CAR-T | NCT02937103 | A Clinical Research of CD123-Targeted CAR-T in Myeloid Malignancies | Leukemia | Recruiting | Southwest Hospital, China | October 18, 2016 |
| CAR-T | NCT02710149 | A Clinical Research of CD20-Targeted CAR-T in B Cell Malignancies | Leukemia, Lymphoma | Recruiting | Southwest Hospital, China | March 16, 2016 |
| CAR-T | NCT04008394 | Anti-CD30 CAR-T Therapy in Patients With Refractory/Relapsed Lymphocyte Malignancies | Adult T-Cell Lymphoma/Leukaemia, Anaplastic Large Cell Lymphoma, Angioimmunoblastic T-cell Lymphoma, NK/T-cell Lymphoma, Peripheral T Cell Lymphoma, Hodgkin Lymphoma | Recruiting | Wuhan Union Hospital, China | July 4, 2019 |
| CAR-T | NCT02954445 | A Clinical Research of BCMA-Targeted CAR-T in B Cell Malignancies | Leukemia, Lymphoma, Multiple Myeloma | Recruiting | Southwest Hospital, China | November 3, 2016 |
| CAR-T | NCT02958410 | A Clinical Research of CD30-Targeted CAR-T in Lymphocyte Malignancies | Leukemia, Lymphoma | Recruiting | Southwest Hospital, China | November 8, 2016 |
| CAR-T | NCT03559439 | CD19-targeting CAR-T Cells in Relapsed or Refractory CD19 Positive B-cell Malignancies | B-cell Malignancy, B-cell Lymphoma, B-Cell Acute Lymphoblastic Leukaemia | Recruiting | Shanghai Tong Ren Hospital | June 18, 2018 |
| CAR-T | NCT03542799 | EGFR-IL12-CART Cells for Patients With Metastatic Colorectal Cancer | Metastatic Colorectal Cancer | Not yet recruiting | Shenzhen Second People's Hospital | May 31, 2018 |
| CAR-T | NCT03152435 | EGFR CAR-T Cells for Patients With Metastatic Colorectal Cancer | EGFR-positive Colorectal Cancer | Recruiting | Shenzhen Second People's Hospital | May 15, 2017 |
| CAR-T | NCT03994705 | Descartes-11 in Multiple Myeloma | Multiple Myeloma | Not yet recruiting | Cartesian Therapeutics | June 21, 2019 |
| CAR-T | NCT03467256 | CD19 T-CAR for Treatment of Children and Young Adults With r/r B-ALL | B-cell Acute Lymphoblastic Leukemia, Acute Lymphocytic Leukemia, Pediatric | Recruiting | Federal Research Institute of Pediatric Hematology, Oncology and Immunology | March 15, 2018 |
| CAR-T | NCT02935153 | A Clinical Research of CD22-Targeted CAR-T in B Cell Malignancies | Leukemia, Lymphoma | Recruiting | Southwest Hospital, China | October 17, 2016 |
| CAR-T | NCT03146533 | CD19 CAR-T Cells for Patients With Relapse and Refractory CD19+ B-cell Lymphoma. | B Cell Lymphoma | Recruiting | Shenzhen Second People's Hospital | May 10, 2017 |
| CAR-T | NCT02737085 | the Sequential Therapy of CD19-targeted and CD20-targeted CAR-T Cell Therapy for Diffuse Large B Cell Lymphoma(DLBCL) | Lymphoma, Large B-Cell, Diffuse | Active, not recruiting | Southwest Hospital, China | April 13, 2016 |
| CAR-T | NCT02443831 | CARPALL: Immunotherapy With CD19 CAR T-cells for CD19+ Haematological Malignancies | Acute Lymphoblastic Leukemia, Burkitt Lymphoma | Recruiting | University College, London | May 14, 2015 |
| CAR-T | NCT03766126 | Lentivirally Redirected CD123 Autologous T Cells in AML | Acute Myeloid Leukemia, in Relapse, Acute Myeloid Leukemia, Adult, Acute Myeloid Leukemia, Refractory | Recruiting | University of Pennsylvania | December 6, 2018 |
| CAR-T | NCT03574168 | CD19-CAR-T Cells in Patients With R/R B-ALL | B-cell Acute Lymphoblastic Leukemia | Recruiting | Bioceltech Therapeutics, Ltd. | June 29, 2018 |
| CAR-T | NCT03747965 | Study of PD-1 Gene-knocked Out Mesothelin-directed CAR-T Cells With the Conditioning of PC in Mesothelin Positive Multiple Solid Tumors | Solid Tumor, Adult | Recruiting | Chinese PLA General Hospital | November 20, 2018 |
| CAR-T | NCT03484702 | Trial to Determine the Efficacy and Safety of JCAR017 in Adult Subjects With Aggressive B-Cell Non-Hodgkin Lymphoma | Lymphoma, Non-Hodgkin | Recruiting | Celgene | April 2, 2018 |
| CAR-T | NCT03796390 | Study Evaluating Safety and Efficacy of CAR-T Cells Targeting CD123 in Patients With Acute Myelocytic Leukemia | Acute Myelocytic Leukemia | Recruiting | Hebei Senlang Biotechnology Inc., Ltd. | January 8, 2019 |
| CAR-T | NCT03121625 | CAR-T Therapy in Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | Leukemia, Lymphoma | Recruiting | Hebei Senlang Biotechnology Inc., Ltd. | April 20, 2017 |
| CAR-T | NCT03312205 | CAR-T Cells for Relapsed or Refractory Haematopoietic and Lymphoid Malignancies | Leukemia, Lymphoma, Multiple Myeloma of Bone (Diagnosis) | Recruiting | Hebei Senlang Biotechnology Inc., Ltd. | October 17, 2017 |
| CAR-T | NCT03885076 | Gamma Delta T Cells in AML | Acute Myeloid Leukemia | Recruiting | Royal Marsden NHS Foundation Trust | March 21, 2019 |
| CAR-T | NCT03258047 | Novel Autologou CAR-T Therapy for Relapsed/Refractory B Cell Lymphoma | B Cell Lymphoma | Active, not recruiting | First Affiliated Hospital of Zhejiang University | August 22, 2017 |
| CAR-T | NCT03191773 | A Study of Anti-CD19 CAR-T Cell Immunotherapy for Refractory /Relapsed B Cell Malignancies | Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Lymphoma | Recruiting | Second Affiliated Hospital of Guangzhou Medical University | June 19, 2017 |
| CAR-T | NCT03960840 | CD19-specific CAR-T Cells in CLL/SLL and DLBCL | Chronic Lymphocytic Leukemia; Small Lymphocytic Lymphoma; Diffuse Large B-cell Lymphoma | Recruiting | Novartis Pharmaceuticals | May 23, 2019 |
| CAR-T | NCT03907527 | Modified Immune Cells (Autologous CAR-T Cells) in Treating Patients With Advanced, Recurrent Platinum Resistant Ovarian, Fallopian Tube or Primary Peritoneal Cancer | Platinum-Resistant Fallopian Tube Carcinoma, Platinum-Resistant Ovarian Carcinoma, Platinum-Resistant Primary Peritoneal Carcinoma, Progressive Disease, Recurrent Fallopian Tube Carcinoma, Recurrent Ovarian Carcinoma, Recurrent Primary Peritoneal Carcinoma, Refractory Fallopian Tube Carcinoma, Refractory Ovarian Carcinoma, Refractory Primary Peritoneal Carcinoma, Stage III Fallopian Tube Cancer AJCC v8, Stage III Ovarian Cancer AJCC v8, Stage III Primary Peritoneal Cancer AJCC v8, Stage IIIA Fallopian Tube Cancer AJCC v8, Stage IIIA Ovarian Cancer AJCC v8, Stage IIIA Primary Peritoneal Cancer AJCC v8, Stage IIIA1 Fallopian Tube Cancer AJCC v8, Stage IIIA1 Ovarian Cancer AJCC v8, Stage IIIA2 Fallopian Tube Cancer AJCC v8, Stage IIIA2 Ovarian Cancer AJCC v8, Stage IIIB Fallopian Tube Cancer AJCC v8, Stage IIIB Ovarian Cancer AJCC v8, Stage IIIB Primary Peritoneal Cancer AJCC v8, Stage IIIC Fallopian Tube Cancer AJCC v8, Stage IIIC Ovarian Cancer AJCC v8, Stage IIIC Primary Peritoneal Cancer AJCC v8, Stage IV Fallopian Tube Cancer AJCC v8, Stage IV Ovarian Cancer AJCC v8, Stage IV Primary Peritoneal Cancer AJCC v8, Stage IVA Fallopian Tube Cancer AJCC v8, Stage IVA Ovarian Cancer AJCC v8, Stage IVA Primary Peritoneal Cancer AJCC v8, Stage IVB Fallopian Tube Cancer AJCC v8, Stage IVB Ovarian Cancer AJCC v8, Stage IVB Primary Peritoneal Cancer AJCC v8 | Recruiting | Fred Hutchinson Cancer Research Center | April 9, 2019 |
| CAR-T | NCT04028479 | The Master Registry of Oncology Outcomes Associated With Testing and Treatment | Adenocarcinoma, Adenocystic Carcinoma, Anal Cancer, Appendix Cancer, Brain Tumor, Glioblastoma, Astrocytoma, Bile Duct Cancer, Cholangiocarcinoma, Bladder Cancer, Bone Cancer, Synovial Sarcoma, Chondrosarcoma, Liposarcoma, Sarcoma, Kaposi, Sarcoma,Soft Tissue, Sarcoma, Osteosarcoma, CNS Cancer, Brain Stem Neoplasms, Breast Cancer, Cervical Cancer, Colorectal Cancer, Rectal Cancer, Colon Cancer, Esophageal Cancer, Esophagus Cancer, Cancer of Colon, Pancreatic Cancer, Cancer of Pancreas, Testis Cancer, Testicular Cancer, Ureter Cancer, Renal Cell Carcinoma, Kidney Cancer, Gestational Trophoblastic Tumor, Head and Neck Neoplasms, Parotid Tumor, Larynx Cancer, Tongue Cancer, Pharynx Cancer, Salivary Gland Cancer, Acute Myeloid Leukemia, Chronic Myeloid Leukemia, Acute Lymphoblastic Leukemia, Multiple Myeloma, Non Hodgkin Lymphoma, Carcinoid Tumor, Lung Cancer, Neuroendocrine Tumors, Mesothelioma, Thyroid Cancer, Parathyroid Neoplasms, Adrenal Cancer, Small Bowel Cancer, Stomach Cancer, Liver Cancer, Hepatic Cancer, Melanoma, Skin Cancer, Unknown Primary Tumors, Uterine Cancer, Fallopian Tube Cancer, Ovarian Cancer, Prostate Cancer, Vaginal Cancer, Penile Cancer, Vulvar Cancer, Waldenstrom Macroglobulinemia, Cancer, Advanced, Thymus Cancer, Nasopharyngeal Carcinoma, Multiple Endocrine Neoplasia, Pheochromocytoma, Small Cell Carcinoma, Pulmonary Carcinoma | Not yet recruiting | Taproot Health | July 22, 2019 |
| CAR-T | NCT03050190 | A Phase I/II Multiple Center Trial of 4SCAR19 Cells in the Treatment of Relapsed and Refractory B Cell Malignancies | B-cell Malignancies | Recruiting | Shenzhen Geno-Immune Medical Institute | February 10, 2017 |
| CAR-T | NCT04016129 | CAR-T Immunotherapy Targeting CD19- ALL | B-cell Leukemia | Recruiting | Shenzhen Geno-Immune Medical Institute | July 11, 2019 |
| CAR-T | NCT03125577 | Combination CAR-T Cell Therapy Targeting Hematological Malignancies | B-cell Malignancies | Recruiting | Shenzhen Geno-Immune Medical Institute | April 24, 2017 |
| CAR-T | NCT03423706 | Clinical Studies of New Model Haploidentical Hematopoietic Stem Cell Transplantation | CD19-chimeric Antigen Receptor T Cells, Relapsed and/or Refractory Acute Lymphoblastic Leukemia, the Haploidentical Hematopoietic Stem Cell Transplantation | Recruiting | First Affiliated Hospital of Harbin Medical University | February 6, 2018 |
| CAR-T | NCT04051216 | The SMART CAR-T Study: Health Information Technology | Leukemia, Acute, Leukemia, Lymphoblastic | Enrolling by invitation | University of Michigan Rogel Cancer Center | August 9, 2019 |
| CAR-T | NCT03601078 | An Efficacy and Safety Study of bb2121 in Subjects With Relapsed and Refractory Multiple Myeloma and in Subjects With High-Risk Multiple Myeloma | Multiple Myeloma | Recruiting | Celgene | July 26, 2018 |
| CAR-T | NCT03975907 | Clinical Trial to Evaluate BCMA Car-T (CT053) in Patients With Relapsed and/or Refractory Multiple Myeloma | Multiple Myeloma | Not yet recruiting | Carsgen Therapeutics, Ltd. | June 5, 2019 |
| CAR-T | NCT03468153 | Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy for CD19+CD22+ Relapsed and Refractory Lymphoma | Lymphoma | Recruiting | Ruijin Hospital | March 16, 2018 |
| CAR-T | NCT03330691 | A Feasibility and Safety Study of Dual Specificity CD19 and CD22 CAR-T Cell Immunotherapy for CD19+CD22+ Leukemia | Leukemia, Lymphoma | Recruiting | Seattle Children's Hospital | November 6, 2017 |
| CAR-T | NCT03684889 | CD19-specific CAR-T Cells With a Fully Human Binding Domain for CD19+ Leukemia or Lymphoma | Leukemia, Lymphoma | Recruiting | Seattle Children's Hospital | September 26, 2018 |
| CAR-T | NCT02721407 | Anti-CD22 CAR-T Therapy for CD19-refractory or Resistant Lymphoma Patients | Recurrent Adult Diffuse Large Cell Lymphoma, Recurrent Follicular Lymphoma, Recurrent Mantle Cell Lymphoma, Stage III/IV Adult Diffuse Large Cell Lymphoma, Stage III/IV Follicular Lymphoma, Stage III/IV Mantle Cell Lymphoma | Recruiting | Xinqiao Hospital of Chongqing | March 29, 2016 |
| CAR-T | NCT02786511 | Longterm Follow-up of Subjects Treated With bb2121 | Multiple Myeloma | Enrolling by invitation | Celgene | June 1, 2016 |
| CAR-T | NCT03208556 | Safety and Efficacy of iPD1 CD19 eCAR T Cells in Relapsed or Refractory B-cell Lymphoma | Relapsed or Refractory B-cell Lymphoma | Recruiting | Peking University | July 5, 2017 |
| CAR-T | NCT02652910 | Memory-enriched CAR-T Cells Immunotherapy for B Cell Lymphoma | Recurrent Adult Diffuse Large Cell Lymphoma, Recurrent Follicular Lymphoma, Recurrent Mantle Cell Lymphoma, Stage III Adult Diffuse Large Cell Lymphoma, Stage III Follicular Lymphoma, Stage III Mantle Cell Lymphoma, Stage IV Adult Diffuse Large Cell Lymphoma, Stage IV Follicular Lymphoma, Stage IV Mantle Cell Lymphoma | Recruiting | Xinqiao Hospital of Chongqing | January 12, 2016 |
| CAR-T | NCT03618381 | EGFR806 CAR-T Cell Immunotherapy for Recurrent/Refractory Solid Tumors in Children and Young Adults | Pediatric Solid Tumor, Germ Cell Tumor, Retinoblastoma, Hepatoblastoma, Wilms Tumor, Rhabdoid Tumor, Carcinoma, Osteosarcoma, Ewing Sarcoma, Rhabdomyosarcoma, Synovial Sarcoma, Clear Cell Sarcoma, Malignant Peripheral Nerve Sheath Tumors, Desmoplastic Small Round Cell Tumor, Soft Tissue Sarcoma, Neuroblastoma | Recruiting | Seattle Children's Hospital | August 7, 2018 |
| CAR-T | NCT02842320 | Targeting Leukemic Stem Cell Expressing the IL-1RAP Protein in Chronic Myelogenous Leukemia (CML) | Chronic Myeloid Leukemia | Recruiting | Centre Hospitalier Universitaire de Besancon | July 22, 2016 |
| CAR-T | NCT03488160 | CAR-T Treatment for Relapse / Refractory Type Safety and Effectiveness of Lymphoma | Lymphoma | Recruiting | Sinobioway Cell Therapy Co., Ltd. | April 4, 2018 |
| CAR-T | NCT03492268 | Safety and Efficacy Evaluation of BCMA-CART for Treating Multiple Myeloma | Multiple Myeloma | Recruiting | Shanghai Bioray Laboratory Inc. | April 10, 2018 |
| CAR-T | NCT03226704 | Leukapheresis for CAR-Therapy Manufacturing | Leukemia, Lymphoma, Acute Lymphoblastic Leukemia, Diffuse Large B-Cell Lymphoma, Non-Hodgkin's Lymphoma | Recruiting | National Cancer Institute (NCI) | July 24, 2017 |
| CAR-T | NCT03672318 | Study of ATLCAR.CD138 Cells for Relapsed/Refractory Multiple Myeloma | Multiple Myeloma, Immune System Diseases | Recruiting | UNC Lineberger Comprehensive Cancer Center | September 14, 2018 |
| CAR-T | NCT03696784 | Anti-CD19 CAR-T Cells With Inducible Caspase 9 Safety Switch for B-cell Lymphoma | Lymphoma, Lymphoma, B-Cell, Immune System Diseases, Immunoproliferative Disorders, Lymphatic Diseases | Recruiting | UNC Lineberger Comprehensive Cancer Center | October 5, 2018 |
| CAR-T | NCT03321123 | MB-CART19.1 in Patients With R/R ALL | Precursor B-Lymphoblastic Lymphoma/Leukaemia Refractory | Not yet recruiting | Shanghai Children's Medical Center | October 25, 2017 |
| CAR-T | NCT03893019 | MB-CART20.1 Melanoma | Melanoma (Skin) | Recruiting | Miltenyi Biotec GmbH | March 27, 2019 |
| CAR-T | NCT04004637 | CD7 CAR-T Cells for Patients With Relapse/Refractory CD7+ NK/T Cell Lymphoma and T-lymphoblastic Lymphoma | T-lymphoblastic Lymphoma, NK/T Cell Lymphoma | Recruiting | PersonGen BioTherapeutics (Suzhou) Co., Ltd. | July 2, 2019 |
| CAR-T | NCT03389035 | Transposon-manipulated Allogeneic CARCIK-CD19 Cells in Pediatric and Adult Patients With r/r ALL Post HSCT | Acute Lymphoblastic Leukemia, in Relapse | Recruiting | Fondazione Matilde Tettamanti Menotti De Marchi Onlus | January 3, 2018 |
| CAR-T | NCT03664635 | MB-CART20.1 Lymphoma | Relapsed or Refractory B-cell Non-Hodgkin's Lymphoma, Non-Hodgkin's Lymphoma, B-cell Lymphoma Refractory, B-cell Lymphoma Recurrent | Recruiting | Miltenyi Biotec GmbH | September 10, 2018 |
| CAR-T | NCT03853616 | MB-CART19.1 r/r CD19+ B-cell Malignancies (BCM) | Acute Lymphoblastic Leukemia Recurrent, B-cell Lymphoma Recurrent, B-cell Lymphoma Refractory, Chronic Lymphocytic Leukemia Recurrent, Chronic Lymphocytic Leukemia Refractory | Recruiting | Miltenyi Biotec GmbH | February 25, 2019 |
| CAR-T | NCT03274219 | Study of bb21217 in Multiple Myeloma | Multiple Myeloma | Recruiting | bluebird bio | September 6, 2017 |
| CAR-T | NCT03117751 | Total Therapy XVII for Newly Diagnosed Patients With Acute Lymphoblastic Leukemia and Lymphoma | Acute Lymphoblastic Leukemia, Acute Lymphoblastic Lymphoma | Recruiting | St. Jude Children's Research Hospital | April 18, 2017 |
| CAR-T | NCT03016377 | Administration of Autologous CAR-T CD19 Antigen With Inducible Safety Switch in Patients With Relapsed/Refractory ALL | Acute Lymphoblastic Leukemia, Immune System Diseases, Immunoproliferative Disorders | Recruiting | UNC Lineberger Comprehensive Cancer Center | January 10, 2017 |
| CAR-T | NCT03289455 | CD19 /22 CAR-T Cells (AUTO3) for the Treatment of B Cell ALL | B Acute Lymphoblastic Leukemia, Recurrent Childhood Acute Lymphoblastic Leukemia, Refractory Childhood Acute Lymphoblastic Leukemia, B-cell Acute Lymphoblastic Leukemia | Recruiting | Autolus Limited | September 21, 2017 |
| CAR-T | NCT03287817 | CD19/22 CAR-T Cells (AUTO3) for the Treatment of Diffuse Large B Cell Lymphoma | Diffuse Large B Cell Lymphoma, Relapsed Diffuse Large B-Cell Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, DLBCL | Recruiting | Autolus Limited | September 19, 2017 |
| CAR-T | NCT03994913 | Clinical Trial to Evaluate CD19 CAR-T (CT032) in Patients With Relapsed and/or Refractory Non-Hodgkin's B Cell Lymphoma | Refractory B-Cell Non-Hodgkin Lymphoma, Relapsed B-cell Non-Hodgkin Lymphoma | Not yet recruiting | Carsgen Therapeutics, Ltd. | June 21, 2019 |
| CAR-T | NCT03661554 | BCMA Nano Antibody CAR-T Cells for Patients With Refractory and Relapsed Multiple Myeloma | Relapsed and Refractory Multiple Myeloma | Recruiting | The Pregene (ShenZhen) Biotechnology Company, Ltd. | September 7, 2018 |
| CAR-T | NCT03690011 | Cell Therapy for High Risk T-Cell Malignancies Using CD7-Specific CAR Expressed On Autologous T Cells | T-cell Acute Lymphoblastic Leukemia, T-cell Acute Lymphoblastic Lymphoma, T-non-Hodgkin Lymphoma | Not yet recruiting | Baylor College of Medicine | October 1, 2018 |
| CAR-T | NCT02813252 | Long-Term Follow-up Study for Patients Previously Treated With JCAR015 | Acute Lymphoblastic Leukemia | Active, not recruiting | Juno Therapeutics, Inc. | June 24, 2016 |
| CAR-T | NCT03642496 | Clinical Study of ET019002-T Cell Therapy for Refractory/Relapsed B-Cell Malignancies | B-Cell Malignancies | Not yet recruiting | First Affiliated Hospital Xi'an Jiaotong University | August 22, 2018 |
| CAR-T | NCT03958656 | T-cells Expressing an Anti-SLAMF7 CAR for Treating Multiple Myeloma | Myeloma-Multiple, Myeloma, Plasma-Cell | Recruiting | National Cancer Institute (NCI) | May 22, 2019 |
| CAR-T | NCT03602612 | T Cells Expressing a Novel Fully-Human Anti-BCMA CAR for Treating Multiple Myeloma | Myeloma-Multiple, Myeloma, Plasma-Cell | Recruiting | National Cancer Institute (NCI) | July 27, 2018 |
| CAR-T | NCT03288493 | P-BCMA-101 Tscm CAR-T Cells in the Treatment of Patients With Multiple Myeloma (MM) | Multiple Myeloma | Recruiting | Poseida Therapeutics, Inc. | September 20, 2017 |
| CAR-T | NCT03563326 | Intraperitoneal Infusion of EpCAM CAR-T Cell in Advanced Gastric Cancer With Peritoneal Metastasis (WCH-GC-CART) | Neoplasm, Stomach, Metastases, Neoplasm, Neoplasm Seeding | Recruiting | Jian-Kun Hu | June 20, 2018 |
| CAR-T | NCT03761056 | Efficacy and Safety of Axicabtagene Ciloleucel as First-Line Therapy in Participants With High-Risk Large B-Cell Lymphoma | B-cell Lymphoma | Recruiting | Kite, A Gilead Company | December 3, 2018 |
| CAR-T | NCT03624036 | Safety and Efficacy of KTE-X19 in Adults With Relapsed/Refractory Chronic Lymphocytic Leukemia | Relapsed/Refractory Chronic Lymphocytic Leukemia | Recruiting | Kite, A Gilead Company | August 9, 2018 |
| CAR-T | NCT02348216 | Safety and Efficacy of KTE-C19 in Adults With Refractory Aggressive Non-Hodgkin Lymphoma | Refractory Diffuse Large B Cell Lymphoma (DLBCL), Relapsed Diffuse Large B-Cell Lymphoma, Transformed Follicular Lymphoma (TFL), Primary Mediastinal B-cell Lymphoma (PMBCL), High Grade B-cell Lymphoma (HGBCL) | Recruiting | Kite, A Gilead Company | January 28, 2015 |
| CAR-T | NCT01860937 | T-Lymphocytes Genetically Targeted to the B-Cell Specific Antigen CD19 in Pediatric and Young Adult Patients With Relapsed B-Cell Acute Lymphoblastic Leukemia | Relapsed B-Cell Acute Lymphoblastic Leukemia | Active, not recruiting | Memorial Sloan Kettering Cancer Center | May 23, 2013 |
| CAR-T/TCR-T | NCT02546167 | CART-BCMA Cells for Multiple Myeloma | Multiple Myeloma | Active, not recruiting | University of Pennsylvania | September 10, 2015 |
| CAR-T | NCT02926833 | Safety and Efficacy of KTE-C19 in Combination With Atezolizumab in Adults With Refractory Diffuse Large B-Cell Lymphoma (DLBCL) | Refractory Diffuse Large B Cell Lymphoma | Active, not recruiting | Kite, A Gilead Company | October 6, 2016 |
| CAR-T | NCT03704298 | Safety and Efficacy of Axicabtagene Ciloleucel in Combination With Utomilumab in Adults With Refractory Large B-cell Lymphoma | Refractory Large B-cell Lymphoma | Recruiting | Kite, A Gilead Company | October 12, 2018 |
| CAR-T | NCT03391466 | Efficacy of Axicabtagene Ciloleucel Compared to Standard of Care Therapy in Subjects With Relapsed/Refractory Diffuse Large B Cell Lymphoma | Relapsed/Refractory Diffuse Large B-Cell Lymphoma (DLBCL) | Recruiting | Kite, A Gilead Company | January 5, 2018 |
| CAR-T | NCT02917083 | CD30 CAR-T Cells, Relapsed CD30 Expressing Lymphoma (RELY-30) | Hodgkin's Lymphoma, Non-Hodgkin Lymphoma | Recruiting | Baylor College of Medicine | September 28, 2016 |
| CAR-T | NCT03271632 | Multi-CAR T Cell Therapy in the Treatment of Multiple Myeloma | Multiple Myeloma | Recruiting | Shenzhen Geno-Immune Medical Institute | September 5, 2017 |
| CAR-T | NCT03310619 | A Safety and Efficacy Trial of JCAR017 Combinations in Subjects With Relapsed/Refractory B-cell Malignancies (PLATFORM) | Lymphoma, Non-Hodgkin, Lymphoma, Large B-Cell, Diffuse, Lymphoma, Follicular | Recruiting | Celgene | October 16, 2017 |
| CAR-T | NCT03389230 | Memory-Enriched T Cells in Treating Patients With Recurrent or Refractory Grade III-IV Glioma | Glioblastoma, HER2/Neu Positive, Malignant Glioma, Recurrent Glioma, Refractory Glioma, WHO Grade III Glioma | Recruiting | City of Hope Medical Center | January 3, 2018 |
| CAR-T | NCT04002401 | Safety and Efficacy of Axicabtagene Ciloleucel in Combination With Either Rituximab or Lenalidomide in Participants With Refractory Large B-Cell Lymphoma (ZUMA-14) | Refractory Large B-cell Lymphoma | Not yet recruiting | Gilead Sciences | June 28, 2019 |
| CAR-T | NCT03331198 | Study Evaluating Safety and Efficacy of JCAR017 in Subjects With Relapsed or Refractory Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL) | Leukemia, Lymphocytic, Chronic, B-Cell, Lymphoma, Small Lymphocytic | Recruiting | Juno Therapeutics, Inc. | November 6, 2017 |
| CAR-T | NCT03483103 | Lisocabtagene Maraleucel (JCAR017) as Second-Line Therapy (TRANSCEND-PILOT-017006) | Lymphoma, Non-Hodgkin, Lymphoma, Nonhodgkin, Lymphoma, B-Cell, Lymphoma, Large B-Cell, Diffuse | Recruiting | Juno Therapeutics, Inc. | March 30, 2018 |
| CAR-T | NCT03744676 | A Safety Trial of Lisocabtagene Maraleucel (JCAR017) for Relapsed and Refractory (R/R) B-cell Non-Hodgkin Lymphoma (NHL) in the Outpatient Setting (TRANSCEND - OUTREACH) | Lymphoma, Non-Hodgkin, Lymphoma, Lymphoma, B-Cell, Lymphoma, Large B-Cell, Diffuse, Neoplasms, Neoplasms by Histologic Type, Lymphoproliferative Disorders, Lymphatic Diseases, Immunoproliferative Disorders, Immune System Disorder | Recruiting | Juno Therapeutics, Inc. | November 16, 2018 |
| CAR-T | NCT03568461 | Efficacy and Safety of Tisagenlecleucel in Adult Patients With Refractory or Relapsed Follicular Lymphoma | Follicular Lymphoma | Recruiting | Novartis Pharmaceuticals | June 25, 2018 |
| CAR-T | NCT02445248 | Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients | Diffuse Large B-cell Lymphoma (DLBCL) | Active, not recruiting | Novartis Pharmaceuticals | May 15, 2015 |
| CAR-T | NCT03181126 | A Study of Venetoclax in Combination With Navitoclax and Chemotherapy in Subjects With Relapsed/Refractory Acute Lymphoblastic Leukemia or Relapsed/Refractory Lymphoblastic Lymphoma | Acute Lymphoblastic Leukemia (ALL), Lymphoblastic Lymphoma | Recruiting | AbbVie | June 8, 2017 |
| CAR-T | NCT03651128 | Efficacy and Safety Study of bb2121 Versus Standard Triplet Regimens in Subjects With Relapsed and Refractory Multiple Myeloma (RRMM) | Multiple Myeloma | Recruiting | Celgene | August 29, 2018 |
| TCR-T | NCT03925896 | Phase I Trial of LMP2 Antigen-specific TCR T-cell Therapy for Recurrent and Metastatic NPC Patients | Nasopharyngeal Carcinoma | Recruiting | Sun Yat-sen University | April 24, 2019 |
| TCR-T | NCT03778814 | TCR-T Cell for Immunotherapy of Lung Cancer and Other Solid Tumors | Nonsmall Cell Lung Cancer, Solid Tumor, Adult | Recruiting | Second Affiliated Hospital of Guangzhou Medical University | December 18, 2018 |
| TCR-T | NCT03891706 | Individualized Tumor Specific TCR- T Cells in the Treatment of Advanced Solid Tumors | Lung Cancer, Melanoma | Recruiting | Guangzhou FineImmune Biotechnology Co., LTD. | March 27, 2019 |
| TCR-T | NCT04044950 | A Phase II Study of Neoadjuvant E7 TCR T Cell Immunotherapy for Borderline Resectable and Unresectable Stage I HPV-Associated Oropharyngeal Cancer | Papillomavirus Infections, Oropharyngeal Neoplasms | Not yet recruiting | National Cancer Institute (NCI) | August 5, 2019 |
| TCR-T | NCT03578406 | HPV-E6-Specific Anti-PD1 TCR-T Cells in the Treatment of HPV-Positive NHSCC or Cervical Cancer | Cervical Cancer, Head and Neck Squamous Cell Carcinoma | Recruiting | Xinqiao Hospital of Chongqing | July 6, 2018 |
| TCR-T | NCT03648697 | EBV-TCR-T(YT-E001)for Patients With EBV-positive Recurrent or Metastatic NPC | Nasopharyngeal Carcinoma | Not yet recruiting | Fujian Cancer Hospital | August 27, 2018 |
| TCR-T | NCT04015336 | E7 TCR Cell Induction Immunotherapy for Stage II and Stage III HPV-Associated Oropharyngeal Cancer | Papillomavirus Infections, Oropharyngeal Neoplasms | Recruiting | National Cancer Institute (NCI) | July 11, 2019 |
| TCR-T | NCT03326921 | HA-1 T TCR T Cell Immunotherapy for the Treating of Patients With Relapsed or Refractory Acute Leukemia After Donor Stem Cell Transplant | HLA-A*0201 HA-1 Positive Cells Present, Juvenile Myelomonocytic Leukemia, Recurrent Acute Biphenotypic Leukemia, Recurrent Acute Undifferentiated Leukemia, Recurrent Childhood Acute Lymphoblastic Leukemia, Recurrent Childhood Acute Myeloid Leukemia, Refractory Acute Lymphoblastic Leukemia, Refractory Adult Acute Lymphoblastic Leukemia, Allogeneic Hematopoietic Stem Cell Transplantation Recipient, Blast Phase Chronic Myelogenous Leukemia, BCR-ABL1 Positive, Chronic Myelomonocytic Leukemia, Donor, Recurrent Blastic Plasmacytoid Dendritic Cell Neoplasm, Recurrent Myelodysplastic Syndrome, Refractory Blastic Plasmacytoid Dendritic Cell Neoplasm, Refractory Myelodysplastic Syndrome, Acute Undifferentiated Leukemia, Blast Cells Present, Blastic Plasmacytoid Dendritic Cell Neoplasm, Blasts 5 Percent or More of Bone Marrow Nucleated Cells, Minimal Residual Disease, Mixed Phenotype Acute Leukemia, Recurrent Chronic Myelogenous Leukemia, BCR-ABL1 Positive, Refractory Chronic Myelogenous Leukemia, BCR-ABL1 Positive, Recurrent Acute Lymphoblastic Leukemia, Recurrent Acute Myeloid Leukemia | Recruiting | Fred Hutchinson Cancer Research Center | October 31, 2017 |
| TCR-T | NCT02719782 | A Study of TCR-Redirected T Cell Infusion in Subject With Recurrent HBV-related HCC Post Liver Transplantation | Recurrent Hepatocellular Carcinoma | Recruiting | Lion TCR Pte. Ltd. | March 25, 2016 |
| TCR-T | NCT03937791 | Immunotherapy With E7 T Cell Receptor T Cells for Vulvar High-Grade Squamous Intraepithelial Lesions | Squamous Lntraepithelial Lesions of Vulva, Neoplasms, Squamous Cell, Vulvar HSIL | Recruiting | National Cancer Institute (NCI) | May 6, 2019 |
| TCR-T | NCT02858310 | E7 TCR T Cells for Human Papillomavirus-Associated Cancers | Papillomavirus Infections, Cervical Intraepithelial Neoplasia, Carcinoma In Situ, Vulvar Neoplasms, Vulvar Diseases | Recruiting | National Cancer Institute (NCI) | August 8, 2016 |
| TCR-T | NCT03029273 | NY-ESO-1 TCR (TAEST16001)for Patients With Advanced NSCLC | Lung Cancer, Nonsmall Cell, Recurrent | Recruiting | Guangzhou Institute of Respiratory Disease | January 24, 2017 |
| TCR-T | NCT03970382 | A Study of Gene Edited Autologous Neoantigen Targeted TCR T Cells With or Without Anti-PD-1 in Patients With Solid Tumors | Solid Tumor | Recruiting | PACT Pharma, Inc. | May 31, 2019 |
| TCR-T | NCT03747484 | Gene-Modified Immune Cells (FH-MCVA2TCR) in Treating Patients With Metastatic or Unresectable Merkel Cell Cancer | Other Skin | Recruiting | Fred Hutchinson Cancer Research Center | November 20, 2018 |
| TCR-T | NCT03392545 | Combination of Immunization and Radiotherapy for Malignant Gliomas (InSituVac1) | High Grade Glioma, Glioblastoma, Glioma of Brainstem, Glioma, Malignant | Recruiting | Beijing Tiantan Hospital | January 8, 2018 |
| TCR-T | NCT03691376 | NY-ESO-1 TCR Engineered T Cell and HSC After Melphalan Conditioning Regimen in Treating Participants With Recurrent or Refractory Ovarian, Fallopian Tube, or Primary Peritoneal Cancer | HLA-A*0201 Positive Cells Present, HLA-DP4 Positive Cells Present, Platinum-Resistant Ovarian Carcinoma, Recurrent Fallopian Tube Carcinoma, Recurrent Ovarian Carcinoma, Recurrent Primary Peritoneal Carcinoma, Refractory Fallopian Tube Carcinoma, Refractory Ovarian Carcinoma, Refractory Primary Peritoneal Carcinoma | Recruiting | Roswell Park Cancer Institute | October 1, 2018 |
| TCR-T | NCT02686372 | TCR-Redirected T Cell Infusions to Prevent Hepatocellular Carcinoma Recurrence Post Liver Transplantation | Hepatocellular Carcinoma | Recruiting | Lion TCR Pte. Ltd. | February 19, 2016 |
| TCR-T | NCT03462316 | NY-ESO-1-specific T Cell Receptor (TCR) T Cell in Sarcoma | Bone Sarcoma, Soft Tissue Sarcoma | Recruiting | Sun Yat-sen University | March 12, 2018 |
| TCR-T | NCT03899415 | TCR-Redirected T Cells Therapy in Patient With HBV Related HCC | Hepatocellular Carcinoma | Recruiting | Beijing 302 Hospital | April 2, 2019 |
| TCR-T | NCT03912831 | Safety and Efficacy of KITE-439 in HLA-A*02:01+ Adults With Relapsed/Refractory HPV16+ Cancers | Human Papillomavirus (HPV) 16+ Relapsed/Refractory Cancer | Recruiting | Kite, A Gilead Company | April 11, 2019 |
| TCR-T | NCT03391791 | Long Term Follow up of Subjects Exposed to Genetically Engineered T Cell Receptors | Solid and Hematological Malignancies | Enrolling by invitation | Adaptimmune | January 5, 2018 |
| TCR-T | NCT03301168 | Study of Gene Modified Donor T-cells Following TCR Alpha Beta Positive Depleted Stem Cell Transplant | Acute Lymphoblastic Leukemia, Leukemia, Acute Myeloid (AML), Child, Lymphoma, Non-Hodgkin, Myelodysplastic Syndromes, Primary Immune Deficiency Disorder, Osteopetrosis, Cytopenia, Hemoglobinopathy in Children, Anemia, Aplastic | Active, not recruiting | Bellicum Pharmaceuticals | October 4, 2017 |
| TCR-T | NCT02654821 | Study With T-cel Receptor Gene Therapy in Metastatic Melanoma | Stage IV Skin Melanoma, Eye; Melanoma | Active, not recruiting | The Netherlands Cancer Institute | January 13, 2016 |
| TCR-T | NCT03717480 | Ex Vivo TCR αβ T Cell Depletion for Graft-Versus-Host Disease Prophylaxis in Mismatched Donor Peripheral Blood Stem Cell Transplantation for Hematologic Malignancies | Hematologic Malignancy | Recruiting | Dana-Farber Cancer Institute | October 24, 2018 |
| TCR-T | NCT02457650 | T Cell Receptor-transduced T Cells Targeting NY-ESO-1 for Treatment of Patients With NY-ESO-1- Expressing Malignancies | Bladder Carcinoma, Breast Cancer, Esophagus Carcinoma, Lung Cancer, Melanoma, Multiple Myeloma, Neuroblastoma, Ovarian Cancer, Synovial Sarcoma, Other Metastatic Solid Cancers | Recruiting | Shenzhen Second People's Hospital | May 29, 2015 |
| TCR-T | NCT03939585 | Preemptive Infusion of Donor Lymphocytes Depleted of TCR + T Cells + CD19+ B Cells Following ASCT | Allogeneic Stem Cell Transplant Candidate, Acute Myeloid/Lymphoblastic Leukemia, Myelodysplastic Syndrome, Myeloproliferative Neoplasm, Lymphoproliferative Disorders | Not yet recruiting | Case Comprehensive Cancer Center | May 7, 2019 |
| TCR-T | NCT03971747 | AFP Specific T Cell Receptor Transduced T Cells Injection(C-TCR055) in Unresectable Hepatocellular Carcinoma | Hepatocellular Carcinoma | Not yet recruiting | Cellular Biomedicine Group Ltd. | June 3, 2019 |
| TCR-T | NCT02065869 | Safety Study of Gene Modified Donor T-cells Following TCR Alpha Beta Depleted Stem Cell Transplant | Acute Lymphoblastic Leukemia, Leukemia, Acute Myeloid (AML), Child, Lymphoma, Non-Hodgkin, Myelodysplastic Syndrome, Primary Immunodeficiency, Anemia, Aplastic, Osteopetrosis, Hemoglobinopathies, Cytopenia, Fanconi Anemia, Diamond Blackfan Anemia, Thalassemia, Anemia, Sickle Cell | Active, not recruiting | Bellicum Pharmaceuticals | February 19, 2014 |
| TCR-T | NCT03907852 | Phase 1/2 Trial of TC-210 T Cells in Patients With Advanced Mesothelin-Expressing Cancer | Mesothelioma, Mesothelioma, Malignant, Mesothelioma; Pleura, Mesotheliomas Pleural, Mesothelioma Peritoneum, Cholangiocarcinoma, Cholangiocarcinoma Recurrent, Ovarian Cancer, Non Small Cell Lung Cancer, Non Small Cell Lung Cancer Metastatic | Recruiting | TCR2 Therapeutics | April 9, 2019 |
| TCR-T | NCT03503968 | TCR Modified T Cells MDG1011 in High Risk Myeloid and Lymphoid Neoplasms | Safety, Tolerability, Feasibility, Treatment Efficacy | Recruiting | Medigene AG | April 20, 2018 |
| TCR-T | NCT02650986 | Gene-Modified T Cells in Treating Patients With Locally Advanced or Stage IV Solid Tumors Expressing NY-ES0-1 | Adult Solid Neoplasm | Recruiting | Roswell Park Cancer Institute | January 8, 2016 |
| TCR-T | NCT03399448 | NY-ESO-1-redirected CRISPR (TCRendo and PD1) Edited T Cells (NYCE T Cells) | Multiple Myeloma, Melanoma, Synovial Sarcoma, Myxoid/Round Cell Liposarcoma | Recruiting | University of Pennsylvania | January 16, 2018 |
| TCR-T | NCT03297697 | Minimal Residual Disease in Peripheral T-cell Lymphoma | Peripheral T Cell Lymphoma | Recruiting | Washington University School of Medicine | September 29, 2017 |
| TCR-T | NCT03354390 | HERV-E TCR Transduced Autologous T Cells in People With Metastatic Clear Cell Renal Cell Carcinoma | Kidney Cancer | Recruiting | National Heart, Lung, and Blood Institute (NHLBI) | November 28, 2017 |
| TCR-T | NCT03996018 | T-cell Responses to Concurrent HIV and Herpesvirus Infections | HIV, HIV Infections | Recruiting | St. Jude Children's Research Hospital | June 24, 2019 |
| TCR-T | NCT03686124 | TCR-engineered T Cells in Solid Tumors | Refractory Cancer, Recurrent Cancer, Solid Tumor, Adult, Cancer | Recruiting | Immatics US, Inc. | September 26, 2018 |
| TCR-T | NCT03422224 | ALLoreactive T-Cell receptOr RePertoire in kidnEy tranSplantation | Renal Transplant Rejection, Immune Repertoire, Transplant; Complication, Rejection, Alloreactivity | Recruiting | Medical University of Vienna | February 5, 2018 |
| TCR-T | NCT02870244 | Adoptive T Cell Immunotherapy for Advanced Melanoma Using Engineered Lymphocytes | Melanoma | Recruiting | Loyola University | August 17, 2016 |
| TCR-T | NCT02869217 | Study of TBI-1301 (NY-ESO-1 Specific TCR Gene Transduced Autologous T Lymphocytes) in Patients With Solid Tumors | NY-ESO-1 Expressing Solid Tumors in HLA-A2 Positive Patients, Synovial Sarcoma, Melanoma, Esophageal Cancer, Ovarian Cancer, Lung Cancer, Bladder Cancer, Liver Cancer | Recruiting | University Health Network, Toronto | August 16, 2016 |
| TCR-T | NCT03159585 | To Evaluate the Efficacy of NY-ESO-1-specific T Cell Receptor Affinity Enhancing Specific T Cell in Solid Tumors | Liver Cancer Stage IV, Gastric Cancer Stage IV, Esophageal Cancer, Stage IV, Bone and Soft Tissue Tumors, Breast Cancer Stage IV, Bladder Carcinoma Stage IV, Prostate Carcinoma Stage IV, Thyroid Cancer Stage IV, Ovarian Cancer Stage IV, Solid Tumor | Recruiting | Zhujiang Hospital | May 18, 2017 |
| TCR-T | NCT03236558 | The Role of the Thymus in Type I Diabetes. | Type1 Diabetes | Not yet recruiting | University Hospital, Toulouse | August 2, 2017 |
| TCR-T | NCT03247309 | TCR-engineered T Cells in Solid Tumors With Emphasis on NSCLC and HNSCC (ACTengine) | Solid Tumor, Cancer, Head and Neck Squamous Cell Carcinoma, Non-small Cell Lung Cancer | Recruiting | Immatics US, Inc. | August 11, 2017 |
| TCR-T | NCT03047746 | Unrelated And Partially Matched Related Donor PSCT w/ TCR αβ Depletion for Patients With BMF | Acquired Aplastic Anemia, Paroxysmal Nocturnal Hemoglobinuria, Inherited Bone Marrow Failure Syndromes | Recruiting | Children's Hospital of Philadelphia | February 9, 2017 |
| TCR-T | NCT03579875 | T Cell Receptor α/β TCD HCT in Patients With Fanconi Anemia | Fanconi Anemia, Severe Aplastic Anemia, Myelodysplastic Syndromes | Recruiting | Masonic Cancer Center, University of Minnesota | July 9, 2018 |
| TCR-T | NCT03412877 | Administration of Autologous T-Cells Genetically Engineered to Express T-Cell Receptors Reactive Against Mutated Neoantigens in People With Metastatic Cancer | Glioblastoma, Non-Small Cell Lung Cancer, Ovarian Cancer, Breast Cancer, Gastrointestinal/Genitourinary Cancer | Recruiting | National Cancer Institute (NCI) | January 29, 2018 |
| TCR-T | NCT02366546 | Investigator Initiated Phase 1 Study of TBI-1301 | Solid Tumors | Active, not recruiting | Mie University | February 19, 2015 |
| TCR-T | NCT02096614 | Investigator Initiated Phase 1 Study of TBI-1201 | Solid Tumors | Recruiting | Mie University | March 26, 2014 |
| TCR-T | NCT03709706 | Pilot Immunotherapy Study With Autologous T-cells Specific for New York Esophageal Antigen-1 (NY-ESO-1)/ Cancer-testis Antigen-2 (LAGE-1a)-Positive Advanced Non-small Cell Lung Cancer (NSCLC) Either Alone or in Combination With Pembrolizumab | Neoplasms | Recruiting | GlaxoSmithKline | October 17, 2018 |
| TCR-T | NCT03649529 | Treatment of Malignant Melanoma With GPA-TriMAR-T Cell Therapy | Malignant Melanoma | Recruiting | Timmune Biotech Inc. | August 28, 2018 |
| TCR-T | NCT03017131 | Genetically Modified T Cells and Decitabine in Treating Patients With Recurrent or Refractory Ovarian, Primary Peritoneal, or Fallopian Tube Cancer | Recurrent Fallopian Tube Carcinoma, Recurrent Ovarian Carcinoma, Recurrent Primary Peritoneal Carcinoma | Recruiting | Roswell Park Cancer Institute | January 11, 2017 |
| TCR-T | NCT02588612 | NY-ESO-1ᶜ²⁵⁹T for Advanced NSCLC | Neoplasms | Active, not recruiting | GlaxoSmithKline | October 28, 2015 |
| TCR-T | NCT03132792 | AFPᶜ³³²T in Advanced HCC | Hepatocellular Cancer | Recruiting | Adaptimmune | April 28, 2017 |
| TCR-T | NCT02743611 | Safety & Activity of Controllable PRAME-TCR Therapy in Previously Treated AML/MDS or Metastatic Uveal Melanoma | Acute Myeloid Leukemia, Myelodysplastic Syndrome, Uveal Melanoma | Active, not recruiting | Bellicum Pharmaceuticals | April 19, 2016 |
| TCR-T | NCT03441100 | TCR-engineered T Cells in Solid Tumors Including NSCLC and HCC Patients | Solid Tumor, Adult, Cancer, Hepatocellular Carcinoma, Hepatocellular Cancer, Nonsmall Cell Lung Cancer, Liver Cancer, Lung Cancer | Recruiting | Immatics US, Inc. | February 21, 2018 |
| TCR-T | NCT03615144 | TCR Alpha Beta T-cell and CD19 B-cell Depleted Peripheral Blood Stem Cell Transplantation Using the CliniMACS System for Patients With Non-Malignant Hematologic Disorders From Matched or Mismatched, Related or Unrelated Donors | Non-Malignant Hematologic Disorders | Recruiting | Memorial Sloan Kettering Cancer Center | August 3, 2018 |
| TCR-T | NCT02770820 | Laboratory-Treated (Central Memory/Naive) CD8+ T Cells in Treating Patients With Newly Diagnosed or Relapsed Acute Myeloid Leukemia | Acute Myeloid Leukemia, EBV-Positive Neoplastic Cells Present, HLA-A*0201 Positive Cells Present, Blasts Under 5 Percent of Bone Marrow Nucleated Cells, Elevated WT1 | Active, not recruiting | Fred Hutchinson Cancer Research Center | May 12, 2016 |
| TCR-T | NCT03139370 | Safety and Efficacy of MAGE-A3/A6 T Cell Receptor Engineered T Cells (KITE-718) in HLA-DPB1*04:01 Positive Adults With Advanced Cancers | Solid Tumor | Recruiting | Kite, A Gilead Company | May 3, 2017 |
| TCR-T | NCT03745326 | Administering Peripheral Blood Lymphocytes Transduced With a Murine T-Cell Receptor Recognizing the G12D Variant of Mutated RAS in HLA-A*11:01 Patients | Gastrointestinal Cancer, Pancreatic Cancer, Gastric Cancer, Colon Cancer, Rectal Cancer | Recruiting | National Cancer Institute (NCI) | November 19, 2018 |
| TCR-T | NCT03190941 | Administering Peripheral Blood Lymphocytes Transduced With a Murine T-Cell Receptor Recognizing the G12V Variant of Mutated RAS in HLA-A*11:01 Patients | Pancreatic Cancer, Gastric Cancer, Gastrointestinal Cancer, Colon Cancer, Rectal Cancer | Recruiting | National Cancer Institute (NCI) | June 19, 2017 |
| TCR-T | NCT02408016 | Genetically Modified T Cells in Treating Patients With Stage III-IV Non-small Cell Lung Cancer or Mesothelioma | Advanced Pleural Malignant Mesothelioma, HLA-A*0201 Positive Cells Present, Recurrent Non-Small Cell Lung Carcinoma, Recurrent Pleural Malignant Mesothelioma, Stage III Non-Small Cell Lung Cancer AJCC v7, Stage III Pleural Malignant Mesothelioma AJCC v7, Stage IIIA Non-Small Cell Lung Cancer AJCC v7, Stage IIIB Non-Small Cell Lung Cancer AJCC v7, Stage IV Non-Small Cell Lung Cancer AJCC v7, Stage IV Pleural Malignant Mesothelioma AJCC v7, WT1 Positive | Active, not recruiting | Fred Hutchinson Cancer Research Center | April 3, 2015 |
| TCR-T | NCT03250325 | Study of TBI-1301 (NY-ESO-1 T Cell Receptor Gene Transduced Autologous T Lymphocytes) in Patients With Synovial Sarcoma | Synovial Sarcoma | Active, not recruiting | Takara Bio Inc. | August 15, 2017 |
| TCR-T | NCT03953599 | CD19-Synthetic T Cell Antigen Receptor(STAR)-T in B-cell Malignancies Patients | B Cell Malignancy | Not yet recruiting | Hebei Yanda Ludaopei Hospital | May 16, 2019 |
| TCR-T | NCT01586403 | Transfer of Genetically Engineered Lymphocytes in Melanoma Patients | Melanoma | Active, not recruiting | Loyola University | April 26, 2012 |
| TCR-T | NCT00001350 | Study of Autoimmune Lymphoproliferative Syndrome (ALPS) | Autoimmune Lymphproliferative Syndrome | Recruiting | National Institute of Allergy and Infectious Diseases (NIAID) | November 4, 1999 |
| TCR-T | NCT03240861 | Genetically Engineered PBMC and PBSC Expressing NY-ESO-1 TCR After a Myeloablative Conditioning Regimen to Treat Patients With Advanced Cancer | HLA-A*0201 Positive Cells Present, Locally Advanced Malignant Neoplasm, NY-ESO-1 Positive, Unresectable Malignant Neoplasm | Recruiting | Jonsson Comprehensive Cancer Center | August 7, 2017 |
| TCR-T | NCT01640301 | Laboratory-Treated T Cells in Treating Patients With High-Risk Relapsed Acute Myeloid Leukemia, Myelodysplastic Syndrome, or Chronic Myelogenous Leukemia Previously Treated With Donor Stem Cell Transplant | Recurrent Adult Acute Myeloid Leukemia, Recurrent Childhood Acute Myeloid Leukemia, Secondary Acute Myeloid Leukemia, Therapy-Related Acute Myeloid Leukemia, Donor, Hematopoietic Cell Transplant Recipient, HLA-A*0201 Positive Cells Present, Recurrent Acute Myeloid Leukemia | Active, not recruiting | Fred Hutchinson Cancer Research Center | July 13, 2012 |
| TCR-T | NCT01967823 | T Cell Receptor Immunotherapy Targeting NY-ESO-1 for Patients With NY-ESO-1 Expressing Cancer | Melanoma, Meningioma, Breast Cancer, Non-Small Cell Lung Cancer, Hepatocellular Cancer | Recruiting | National Cancer Institute (NCI) | October 23, 2013 |
| TCR-T | NCT02111850 | T Cell Receptor Immunotherapy Targeting MAGE-A3 for Patients With Metastatic Cancer Who Are HLA-DP0401 Positive | Cervical Cancer, Renal Cancer, Urothelial Cancer, Melanoma, Breast Cancer | Recruiting | National Cancer Institute (NCI) | April 11, 2014 |
| TCR-T | NCT03093688 | Clinical Safty and Efficacy Study of Infusion of iNKT Cells and CD8+T Cells in Patients With Advanced Solid Tumor | Non-small Cell Lung Cancer, Small Cell Lung Cancer, Pancreas Cancer, Hepatocellular Carcinoma, Gastric Cancer, Renal Cell Carcinoma | Recruiting | Shanghai Public Health Clinical Center | March 28, 2017 |
| TCR-T | NCT02652468 | TCR-α/β and CD19 Depleted Stem Cell Grafts From Haplo Donors for HSCT in Relapsed Lymphoma | Lymphoma | Active, not recruiting | University of Wisconsin, Madison | January 11, 2016 |
| TCR-T | NCT03391778 | Long Term Follow-Up of Subjects Exposed to GSK3377794 | Neoplasms | Recruiting | GlaxoSmithKline | January 5, 2018 |
| TCR-T | NCT03733249 | Long Term Follow-up Study for Patients Enrolled on the BP-004 Clinical Study | Acute Lymphoblastic Leukemia, Leukemia, Acute Myeloid (AML), Child, Lymphoma, Non-Hodgkin, Myelodysplastic Syndromes, Primary Immunodeficiency, Anemia, Aplastic, Hemoglobinopathies, Cytopenia, Fanconi Anemia, Diamond Blackfan Anemia, Thalassemia, Anemia, Sickle Cell | Enrolling by invitation | Bellicum Pharmaceuticals | November 7, 2018 |
| TCR-T | NCT02840110 | Long-Term Follow-Up Study of Subjects Treated With ACTR T Cell Product | B Cell Lymphomas, Multiple Myeloma, Solid Tumor, HER-2 Protein Overexpression | Enrolling by invitation | Unum Therapeutics Inc. | July 21, 2016 |
| TCR-T | NCT03849651 | TCRαβ-depleted Progenitor Cell Graft With Additional Memory T-cell DLI, Plus Selected Use of Blinatumomab, in Naive T-cell Depleted Haploidentical Donor Hematopoietc Cell Transplantation for Hematologic Malignancies | Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML), Myelodysplastic Syndromes (MDS), NK-Cell Leukemia, Hodgkin Lymphoma, Non Hodgkin Lymphoma (NHL), Juvenile Myelomonocytic Leukemia (JMML), Chronic Myeloid Leukemia (CML) | Recruiting | St. Jude Children's Research Hospital | February 21, 2019 |
| TCR-T | NCT02646839 | KIR Favorable Mismatched Haplo Transplant and KIR Polymorphism in ALL/AML/MDS Allo-HCT Children | Acute Lymphoblastic Leukemia, Acute Myeloid Leukemia, Myelodysplastic Syndromes | Enrolling by invitation | Michael Pulsipher, MD | January 6, 2016 |
| TCR-T | NCT02942173 | CD45RA Depleted T-cell Infusion for Prevention of Infections After TCRab/CD19-depleted Allo-HSCT | Leukemia, Lymphoma, Myelodysplastic Syndromes, GvHD, Opportunistic Infections, Graft-versus-Host Disease | Recruiting | Federal Research Institute of Pediatric Hematology, Oncology and Immunology | October 21, 2016 |
| TCR-T | NCT00622232 | A Rollover Study for Subjects Who Completed Participation in the VRX496-USA-05-002 Trial | HIV Infections | Active, not recruiting | VIRxSYS Corporation | February 22, 2008 |
| TCR-T | NCT01982890 | Telomeres and T-cell Receptor Excision Circles (TRECs) From Peripheral Blood in Normal Subjects Over Time | Telomere Length in Healthy Controls | Enrolling by invitation | Université de Sherbrooke | November 13, 2013 |
| TCR-T | NCT03132922 | MAGE-A4ᶜ¹º³²T for Multi-Tumor | Urinary Bladder Cancer, Melanoma, Head and Neck Cancer, Ovarian Cancer, Non-Small Cell Lung Cancer, Esophageal Cancer, Gastric Cancer, Synovial Sarcoma, Myxoid Round Cell Liposarcoma | Recruiting | Adaptimmune | April 28, 2017 |
| TCR-T | NCT03603002 | Pathologic and Immunologic Response After Ablative Radiation in Lung Cancer | Non-small Cell Lung Cancer Stage I | Recruiting | Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins | July 27, 2018 |
| TCR-T | NCT03168438 | NY-ESO-1ᶜ²⁵⁹T Alone and in Combination With Pembrolizumab for Multiple Myeloma | Neoplasms | Recruiting | GlaxoSmithKline | May 30, 2017 |
| TCR-T | NCT03921047 | Characterization of T-cell Repertoire in Patients With Acute Myeloid Leukemia Undergoing Donor Stem Cell Transplant | Acute Myeloid Leukemia | Recruiting | University of Southern California | April 19, 2019 |
| TCR-T | NCT02636855 | Screening Protocol for Tumor Antigen Expression Profiling and HLA Typing for Eligibility Determination | Solid and Hematological Malignancies | Recruiting | Adaptimmune | December 22, 2015 |
| TCR-T | NCT02776813 | Study of ACTR087 in Subjects With Relapsed or Refractory B-cell Lymphoma | Lymphoma | Active, not recruiting | Unum Therapeutics Inc. | May 18, 2016 |
| TCR-T | NCT03369353 | Precision Diagnostics in Inflammatory Bowel Disease, Cellular Therapy and Transplantation (The PREDICT Trial) | Graft Vs Host Disease, Inflammatory Bowel Diseases, Functional Gastrointestinal Disorders | Enrolling by invitation | Boston Children's Hospital | December 12, 2017 |
| TCR-T | NCT03265106 | A Clinical Study Evaluating the Safety and Efficacy of BinD19 Treatment in Childhood R/R ALL and Lymphoma Subjects | Relapsed B-cell Acute Lymphoblastic Leukemia, Childhood, Refractory B-cell Acute Lymphoblastic Leukemia, Childhood, Relapsed/Refractory B-cell Lymphoma, Childhood | Recruiting | Shenzhen BinDeBio Ltd. | August 29, 2017 |
| TCR-T | NCT03880279 | Autologous TAC T Cells Targeting CD19 in R/R Large B-Cell Lymphoma | Lymphoma, B-Cell | Not yet recruiting | Triumvira Immunologics, Inc. | March 19, 2019 |
| TCR-T | NCT02774291 | Anti-ESO (Cancer/Test Antigen) mTCR-transduced Autologous Peripheral Blood Lymphocytes and Combination Chemotherapy in Treating Patients With Metastatic Cancer That Expresses NY-ESO-1 | HLA-A2 Positive Cells Present, Metastatic Malignant Neoplasm, Metastatic Malignant Neoplasm in the Brain | Recruiting | Albert Einstein College of Medicine | May 17, 2016 |
| TCR-T | NCT03587285 | A Pilot Study of Hormonal Therapy Combined With Central Memory T Cells (Tcm) for Patients With Advanced Prostate Cancer | Prostatic Neoplasms | Active, not recruiting | Guangzhou University of Traditional Chinese Medicine | July 16, 2018 |
| TCR-T | NCT02133196 | T Cell Receptor Immunotherapy for Patients With Metastatic Non-Small Cell Lung Cancer | Advanced Non-Small Cell Lung Cancer, Squamous Cell Carcinoma, Advanced NSCLC, Adenosquamous Carcinoma, Adenocarcinomas | Recruiting | National Cancer Institute (NCI) | May 7, 2014 |
| TCR-T | NCT03680560 | Study of ACTR T Cell Product in Combination With Trastuzumab in Subjects With HER2-Positive Advanced Solid Tumor Cancers | Solid Tumor, HER-2 Protein Overexpression | Recruiting | Unum Therapeutics Inc. | September 21, 2018 |
| TCR-T | NCT03738228 | Atezolizumab Before and/or With Chemoradiotherapy in Immune System Activation in Patients With Node Positive Stage IB2, II, IIIB, or IVA Cervical Cancer | Cervical Adenocarcinoma, Cervical Adenosquamous Carcinoma, Cervical Squamous Cell Carcinoma, Not Otherwise Specified, Positive Lymph Node, Stage IB2 Cervical Cancer AJCC v8, Stage II Cervical Cancer AJCC v8, Stage IIA Cervical Cancer AJCC v8, Stage IIA1 Cervical Cancer AJCC v8, Stage IIA2 Cervical Cancer AJCC v8, Stage IIB Cervical Cancer AJCC v8, Stage IIIB Cervical Cancer AJCC v8, Stage IVA Cervical Cancer AJCC v8 | Recruiting | National Cancer Institute (NCI) | November 12, 2018 |
| TCR-T | NCT00910650 | Study of Gene Modified Immune Cells in Patients With Advanced Melanoma | Metastatic Melanoma | Active, not recruiting | Jonsson Comprehensive Cancer Center | June 1, 2009 |
| TCR-T | NCT03156101 | A Clinical Study Evaluating the Safety and Efficacy of BinD19 Treatment in R/R ALL and Lymphoma Subjects | Relapsed B-cell Acute Lymphoblastic Leukemia, Refractory B-cell Acute Lymphoblastic Leukemia, Relapsed/Refractory B-cell Lymphoma | Recruiting | Shenzhen BinDeBio Ltd. | May 17, 2017 |
| TCR-T | NCT03118687 | Human Immunome Program | Healthy | Enrolling by invitation | Vanderbilt University Medical Center | April 18, 2017 |
| TCR-T | NCT01744223 | Safety Study of Gene Modified Donor T-cells Following Partially Mismatched Stem Cell Transplant | Acute Lymphoblastic Leukemia, Acute Myelogenous Leukemia, Lymphoma | Active, not recruiting | Bellicum Pharmaceuticals | December 6, 2012 |
| TCR-T | NCT03515551 | Safety and Efficacy of IMCnyeso in Advanced NY-ESO-1 and/or LAGE-1A Positive Cancers | Select Advanced Solid Tumors | Recruiting | Immunocore Ltd | May 3, 2018 |
| TCR-T | NCT01827579 | Infusion of Depleted T Cells Following Unrelated Donor Stem Cell Transplant (ICAT) | Haematological Malignancies | Active, not recruiting | University College, London | April 9, 2013 |
| TCR-T | NCT03619551 | Conditioning SCID Infants Diagnosed Early | SCID | Recruiting | Michael Pulsipher, MD | August 8, 2018 |
| TCR-T | NCT02737046 | Belinostat Therapy With Zidovudine for Adult T-Cell Leukemia-Lymphoma | Adult T-cell Leukemia-Lymphoma, ATLL | Recruiting | University of Miami | April 13, 2016 |
| TCR-T | NCT01884168 | Study of Gene Expression Profiling and Immunological Mechanism Affects the Response of Immunotherapy | Malignant Tumor | Recruiting | Capital Medical University | June 21, 2013 |
| TCR-T | NCT03634683 | A Study of LioCyx in Patient With Recurrent HBV-related HCC Post Liver Transplantation | Recurrent Hepatocellular Carcinoma | Not yet recruiting | Lion TCR Pte. Ltd. | August 16, 2018 |
| TCR-T | NCT03318900 | T-Cell Infusion, Aldesleukin, and Utomilumab in Treating Patients With Recurrent Ovarian Cancer | COL6A3 Positive, HLA-A*0201 Positive Cells Present, PRAME Positive, Recurrent Ovarian Carcinoma | Recruiting | M.D. Anderson Cancer Center | October 24, 2017 |
| TCR-T | NCT03559803 | A Prospective Study of Monitoring Immune Response in Locally Advanced Cervix Cancer | Cervical Cancer | Active, not recruiting | Sichuan University | June 18, 2018 |
| TCR-T | NCT01697527 | Gene and Vaccine Therapy in Treating Patients With Advanced Malignancies | Malignant Neoplasm | Active, not recruiting | Jonsson Comprehensive Cancer Center | October 2, 2012 |
| TCR-T | NCT02210741 | Next-generation Sequencing (NGS) of Peripheral Blood Immune Repertoire in Graves' Disease | Graves Disease | Not yet recruiting | National Taiwan University Hospital | August 7, 2014 |
| TCR-T | NCT02761122 | Implication of Human Papillomavirus (HPV) in Lichen Physiopathology in Human (HPVLichen) | Lichen Planus | Active, not recruiting | Institut Pasteur | May 4, 2016 |
| TCR-T | NCT03879876 | Safety and Efficacy Study of Human T Lymphoid Progenitor (HTLP) Injection After Partially HLA Compatible Allogeneic Hematopoietic Stem Cell Transplantation in SCID Patients | Pediatric Patients, Any Type of Severe Combined Immunodeficiency (SCID), Partial HLA Incompatible Allogeneic Hematopoietic Stem Cell Transplantation (HSCT) | Not yet recruiting | Assistance Publique - Hôpitaux de Paris | March 19, 2019 |
| TCR-T | NCT03340155 | Mechanisms of Action of Photo(Chemo)Therapy in Skin Diseases | Psoriasis, Cutaneous T Cell Lymphoma, Lymphoproliferative Disorders, Eczema, Lichen Planus, Prurigo, Pruritus, Polymorphic Light Eruption, Graft Vs Host Disease, Mastocytosis, Vitiligo | Recruiting | Medical University of Graz | November 13, 2017 |
| TCR-T | NCT02590328 | Neonatal Screening of Severe Combined Immunodeficiencies | Severe Combined Immunodeficiency, Neonatal Screening | Recruiting | Children's Hospital of Fudan University | October 29, 2015 |
| TCR-T | NCT03551795 | Clinical Research on Comprehensive Treatment of Tuberculosis With Malignant Solid Tumor | Malignant Solid Tumor | Recruiting | Xiaoyan Zhang | June 11, 2018 |
| TCR-T | NCT02102893 | Gastroenteral-Pancreatic Neuroendocrine Tumors in Taiwan | This Study Plans to Include 600 GEP-NET Patients. | Enrolling by invitation | National Health Research Institutes, Taiwan | April 3, 2014 |
| TCR-T | NCT01906632 | Gene Expression Profiling of Malignant Tumor Predict the Therapeutic Response of DC-CIK Immunotherapy | Malignant Tumor | Recruiting | Capital Medical University | July 24, 2013 |
| TCR-T | NCT01050296 | Molecular Analysis Of Solid Tumors | Pediatric Solid Tumors | Recruiting | St. Jude Children's Research Hospital | January 15, 2010 |
| TCR-T | NCT03967223 | Master Protocol to Assess the Safety and Antitumor Activity of Genetically Engineered T Cells in NY-ESO-1 and/or LAGE-1a Positive Solid Tumors | Neoplasms | Not yet recruiting | GlaxoSmithKline | May 30, 2019 |
| TCR-T | NCT03407170 | Immunologic Determinants of Response to Pembrolizumab (MK-3475) in Advanced Melanoma (MK-3475-161/KEYNOTE-161) | Advanced Melanoma | Active, not recruiting | Merck Sharp & Dohme Corp. | January 23, 2018 |
| TCR-T | NCT03523429 | PETHEMA-BLIN-01/PET069014 (BLIN-01) | Philadelphia Chromosome-negative or BCR-ABL-negative, CD19-positive ALL | Recruiting | PETHEMA Foundation | May 14, 2018 |
| TCR-T | NCT03129061 | Study to Evaluate Immunological Response to PD-1 Inhibition in Squamous Cell Carcinoma of the Head and Neck (SCCHN) | Squamous Cell Carcinoma of the Head and Neck | Recruiting | CellSight Technologies, Inc. | April 26, 2017 |
| TCR-T | NCT03601286 | Lentiviral Gene Therapy for X-linked Severe Combined Immunodeficiency | Severe Combined Immunodeficiency, X-Linked | Recruiting | Great Ormond Street Hospital for Children NHS Foundation Trust | July 26, 2018 |
| TCR-T | NCT01343043 | A Pilot Study of Genetically Engineered NY-ESO-1 Specific NY-ESO-1ᶜ²⁵⁹T in HLA-A2+ Patients With Synovial Sarcoma | Neoplasms | Active, not recruiting | GlaxoSmithKline | April 27, 2011 |
| TCR-T | NCT03948763 | A Study of mRNA-5671/V941 as Monotherapy and in Combination With Pembrolizumab (V941-001) | Neoplasms, Carcinoma, Non-Small-Cell Lung, Pancreatic Neoplasms, Colorectal Neoplasms | Recruiting | Merck Sharp & Dohme Corp. | May 14, 2019 |
| TCR-T | NCT02992743 | A Pilot Study of NY-ESO-1ᶜ²⁵⁹T Cells in Subjects With Advanced Myxoid/ Round Cell Liposarcoma | Neoplasms | Recruiting | GlaxoSmithKline | December 14, 2016 |
| TCR-T | NCT03852823 | Study of Recombinant Human Anti-PD-1 Monoclonal Antibody for Patients With Advanced Solid Tumors | SG001, PD-1, Advanced Solid Tumors | Not yet recruiting | CSPC ZhongQi Pharmaceutical Technology Co., Ltd. | February 25, 2019 |
| TCR-T | NCT02989064 | MAGE-A10ᶜ⁷⁹⁶T for Urothelial Cancer, Melanoma or Head and Neck Cancers | Urothelial Carcinoma, Head and Neck Cancer, Melanoma, Bladder Urothelial Carcinoma | Recruiting | Adaptimmune | December 12, 2016 |
| TCR-T | NCT03397355 | Monitoring the Changes of Tumor-related Biomarkers Before and After Pulmonary Nodule Biopsy. | Lung Cancer | Recruiting | China-Japan Friendship Hospital | January 12, 2018 |
| TCR-T | NCT02592577 | MAGE A10ᶜ⁷⁹⁶T for Advanced NSCLC | Non-Small Cell Lung Cancer, Carcinoma | Recruiting | Adaptimmune | October 30, 2015 |
| CAR-T/TCR-T | NCT02706392 | Genetically Modified T-Cell Therapy in Treating Patients With Advanced ROR1+ Malignancies | Estrogen Receptor Negative, HER2/Neu Negative, Progesterone Receptor Negative, Recurrent Adult Acute Lymphoblastic Leukemia, Recurrent Mantle Cell Lymphoma, Refractory Chronic Lymphocytic Leukemia, Stage IV Breast Cancer AJCC v6 and v7, Stage IV Non-Small Cell Lung Cancer AJCC v7, Triple-Negative Breast Carcinoma | Recruiting | Fred Hutchinson Cancer Research Center | March 11, 2016 |
| CAR-T/TCR-T | NCT03545815 | Study of CRISPR-Cas9 Mediated PD-1 and TCR Gene-knocked Out Mesothelin-directed CAR-T Cells in Patients With Mesothelin Positive Multiple Solid Tumors. | Solid Tumor, Adult | Recruiting | Chinese PLA General Hospital | June 4, 2018 |
| CAR-T/TCR-T | NCT00085930 | Blood T-Cells and EBV Specific CTLs Expressing GD-2 Specific Chimeric T Cell Receptors to Neuroblastoma Patients | Neuroblastoma | Active, not recruiting | Baylor College of Medicine | June 21, 2004 |
| CAR-T/TCR-T | NCT00924326 | CAR-T Cell Receptor Immunotherapy for Patients With B-cell Lymphoma | Primary Mediastinal B-cell Lymphoma, Diffuse, Large B-cell Lymphoma, Diffuse Large B-Cell Lymphoma Transformed From Follicular Lymphoma, Mantle Cell | Active, not recruiting | National Cancer Institute (NCI) | June 18, 2009 |
| CAR-T/TCR-T | NCT03904069 | Study Evaluating the Safety, Tolerability, and Efficacy of FLT3 CAR-T AMG 553 in FLT3-positive Relapsed/Refractory AML | Relapsed/Refractory Acute Myeloid Leukemia | Not yet recruiting | Amgen | April 4, 2019 |
| CAR-T/TCR-T | NCT01430390 | In Vitro Expanded Allogeneic Epstein-Barr Virus Specific Cytotoxic T-Lymphocytes (EBV-CTLs) Genetically Targeted to the CD19 Antigen in B-cell Malignancies | Acute Lymphocytic Leukemia, Lymphoma | Recruiting | Memorial Sloan Kettering Cancer Center | September 8, 2011 |
| CAR-T/TCR-T | NCT03166878 | A Study Evaluating UCART019 in Patients With Relapsed or Refractory CD19+ Leukemia and Lymphoma | B Cell Leukemia, B Cell Lymphoma | Recruiting | Chinese PLA General Hospital | May 25, 2017 |
| CAR-T/TCR-T | NCT03980691 | Effect of Chidamide Combined With CAT-T or TCR-T Cell Therapy on HIV-1 Latent Reservoir | HIV/AIDS | Recruiting | Guangzhou 8th People's Hospital | June 10, 2019 |
| CAR-T/TCR-T | NCT02624258 | Pilot Study of Non-Viral, RNA-Redirected Autologous T Cells in Patients With Refractory or Relapsed Hodgkin Lymphoma | Hodgkin Lymphoma | Active, not recruiting | University of Pennsylvania | December 8, 2015 |
| CAR-T/TCR-T | NCT02030834 | Phase IIa Study of Redirected Autologous T Cells Engineered to Contain Anti-CD19 Attached to TCRz and 4-Signaling Domains in Patients With Chemotherapy Relapsed or Refractory CD19+ Lymphomas | Non-Hodgkins Lymphoma (NHL) Patients, With CD19+B Cell Lymphomas | Active, not recruiting | University of Pennsylvania | January 9, 2014 |
| CAR-T/TCR-T | NCT03740256 | Binary Oncolytic Adenovirus in Combination With HER2-Specific CAR VST, Advanced HER2 Positive Solid Tumors (VISTA) | Bladder Cancer, Head and Neck Squamous Cell Carcinoma, Cancer of the Salivary Gland, Lung Cancer, Breast Cancer, Gastric Cancer, Esophageal Cancer, Colorectal Cancer, Pancreatic Adenocarcinoma | Not yet recruiting | Baylor College of Medicine | November 14, 2018 |
| CAR-T/TCR-T | NCT03941626 | Autologous CAR-T/TCR-T Cell Immunotherapy for Solid Malignancies | Esophagus Cancer, Hepatoma, Glioma, Gastric Cancer | Recruiting | Shenzhen BinDeBio Ltd. | May 8, 2019 |
| CAR-T/TCR-T | NCT03638206 | Autologous CAR-T/TCR-T Cell Immunotherapy for Malignancies | B-cell Acute Lymphoblastic Leukemia, Lymphoma, Myeloid Leukemia, Multiple Myeloma, Hepatoma, Gastric Cancer, Pancreatic Cancer, Mesothelioma, Colorectal Cancer, Esophagus Cancer, Lung Cancer, Glioma, Melanoma, Synovial Sarcoma, Ovarian Cancer, Renal Carcinoma | Recruiting | Shenzhen BinDeBio Ltd. | August 20, 2018 |
| CAR-T/TCR-T | NCT01192464 | EBV CTLs Expressing CD30 Chimeric Receptors For CD 30+ Lymphoma | Hodgkin's Lymphoma, Non-Hodgkin's Lymphoma | Active, not recruiting | Baylor College of Medicine | September 1, 2010 |
| CAR-NK | NCT03692767 | Study of Anti-CD22 CAR NK Cells in Relapsed and Refractory B Cell Lymphoma | Refractory B-Cell Lymphoma | Not yet recruiting | Allife Medical Science and Technology Co., Ltd. | October 2, 2018 |
| CAR-NK | NCT03690310 | Study of Anti-CD19 CAR NK Cells in Relapsed and Refractory B Cell Lymphoma | Refractory B-Cell Lymphoma | Not yet recruiting | Allife Medical Science and Technology Co., Ltd. | October 1, 2018 |
| CAR-NK | NCT03692663 | Study of Anti-PSMA CAR NK Cell in Castration-Resistant Prostate Cancer | Castration-resistant Prostate Cancer | Not yet recruiting | Allife Medical Science and Technology Co., Ltd. | October 2, 2018 |
| CAR-NK | NCT03692637 | Study of Anti-Mesothelin Car NK Cells in Epithelial Ovarian Cancer | Epithelial Ovarian Cancer | Not yet recruiting | Allife Medical Science and Technology Co., Ltd. | October 2, 2018 |
| CAR-NK | NCT03415100 | Pilot Study of NKG2D-Ligand Targeted CAR-NK Cells in Patients With Metastatic Solid Tumours | Solid Tumours | Recruiting | The Third Affiliated Hospital of Guangzhou Medical University | January 30, 2018 |
| CAR-NK | NCT03940820 | Clinical Research of ROBO1 Specific CAR-NK Cells on Patients With Solid Tumors | Solid Tumor | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | May 7, 2019 |
| CAR-NK | NCT03940833 | Clinical Research of Adoptive BCMA CAR-NK Cells on Relapse/Refractory MM | Multiple Myeloma | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | May 7, 2019 |
| CAR-NK | NCT03824964 | Study of Anti-CD19/CD22 CAR NK Cells in Relapsed and Refractory B Cell Lymphoma | Refractory B-Cell Lymphoma | Not yet recruiting | Allife Medical Science and Technology Co., Ltd. | January 31, 2019 |
| CAR-NK | NCT03941457 | Clinical Research of ROBO1 Specific BiCAR-NK Cells on Patients With Pancreatic Cancer | Pancreatic Cancer | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | May 8, 2019 |
| CAR-NK | NCT03931720 | Clinical Research of ROBO1 Specific BiCAR-NK/T Cells on Patients With Malignant Tumor | Malignant Tumor | Recruiting | Asclepius Technology Company Group (Suzhou) Co., Ltd. | April 30, 2019 |
| CAR-NK | NCT03579927 | CAR.CD19-CD28-zeta-2A-iCasp9-IL15-Transduced Cord Blood NK Cells, High-Dose Chemotherapy, and Stem Cell Transplant in Treating Participants With B-cell Lymphoma | CD19 Positive, Mantle Cell Lymphoma, Recurrent Diffuse Large B-Cell Lymphoma, Recurrent Follicular Lymphoma, Refractory B-Cell Non-Hodgkin Lymphoma, Refractory Diffuse Large B-Cell Lymphoma, Refractory Follicular Lymphoma | Not yet recruiting | M.D. Anderson Cancer Center | July 9, 2018 |
| CAR-NK | NCT03056339 | Umbilical & Cord Blood (CB) Derived CAR-Engineered NK Cells for B Lymphoid Malignancies | B-Lymphoid Malignancies, Acute Lymphocytic Leukemia, Chronic Lymphocytic Leukemia, Non-hodgkin Lymphoma | Recruiting | M.D. Anderson Cancer Center | February 17, 2017 |
| CAR-NK | NCT04033302 | Multi-CAR T Cell Therapy Targeting CD7-positive Malignancies | T-cell Acute Lymphoblastic Leukemia, T-cell Acute Lymphoblastic Lymphoma, Acute Myeloid Leukemia, NK Cell Lymphoma | Recruiting | Shenzhen Geno-Immune Medical Institute | July 26, 2019 |
| CAR-NK | NCT03049449 | T Cells Expressing a Fully-Human Anti-CD30 Chimeric Antigen Receptor for Treating CD30-Expressing Lymphomas | Lymphoma, Large-Cell, Anaplasitc, Hodgkin Disease, Lymphoma, Hodgkins, Enteropathy-Associated T-Cell Lymphoma | Active, not recruiting | National Cancer Institute (NCI) | February 10, 2017 |
| CAR-NK | NCT04008394 | Anti-CD30 CAR-T Therapy in Patients With Refractory/Relapsed Lymphocyte Malignancies | Adult T-Cell Lymphoma/Leukaemia, Anaplastic Large Cell Lymphoma, Angioimmunoblastic T-cell Lymphoma, NK/T-cell Lymphoma, Peripheral T Cell Lymphoma, Hodgkin Lymphoma | Recruiting | Wuhan Union Hospital, China | July 4, 2019 |
| CAR-NKT | NCT03882840 | Induced-T Cell Like NK Cellular Immunotherapy for Cancer Lack of MHC-I | Anti-cancer Cell Immunotherapy, T Cell and NK Cell | Recruiting | Second Affiliated Hospital of Guangzhou Medical University | March 20, 2019 |
| CAR-NKT | NCT03294954 | GD2 Specific CAR and Interleukin-15 Expressing Autologous NKT Cells to Treat Children With Neuroblastoma | Neuroblastoma | Recruiting | Baylor College of Medicine | September 27, 2017 |
| CAR-NKT | NCT03774654 | CD19.CAR Allogeneic NKT for Patients With Relapsed or Refractory B-Cell Malignancies (ANCHOR) | Refractory B-Cell Non-Hodgkin Lymphoma, Refractory B-Cell Small Lymphocytic Lymphoma, Relapsed Adult ALL, Relapsed CLL, Relapsed Non Hodgkin Lymphoma | Not yet recruiting | Baylor College of Medicine | December 13, 2018 |
| CAR-Macrophage | NCT03608618 | Intraperitoneal MCY-M11 (Mesothelin-targeting CAR) for Treatment of Advanced Ovarian Cancer and Peritoneal Mesothelioma | Peritoneal Mesothelioma, Fallopian Tube Adenocarcinoma, Adenocarcinoma of the Ovary, Primary Peritoneal Carcinoma | Recruiting | MaxCyte, Inc. | August 1, 2018 |
 Vaccine usually refers to biological products made from pathogenic microorganisms providing active acquired immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. With the deeper understanding of the immune system and advances in biotechnology, scientists discovered a novel form of vaccines - cell vaccines, which are referred to dead or living cells that are used to induce an immune response in the recipient, with the aim of protecting them from future disease or infection. Compared to non-immune cells, immune cells are better choice in the production of cell vaccines for their significant roles in the adaptive immune responses. T-cell vaccination is immunization with inactivated autoreactive T cells (T cell vaccine, TCV). The concept of T-cell vaccination is, at least partially, analogous to classical vaccination against infectious disease. However, the agents to be eliminated or neutralized are not foreign microbial agents but a pathogenic autoreactive T-cell population. Research on T-cell vaccination so far has focused mostly on multiple sclerosis and to a lesser extent on rheumatoid arthritis, Crohn's disease and AIDS. Another immune cells that are widely studied for vaccination are dendritic cells (DCs). DC vaccine has been introduced as a new therapeutic strategy in cancer patients. DC-based immunotherapy is safe and can promote antitumor immune responses and prolong survival of cancer patients. DC-based immunotherapy approach can be employed in a couple of ways: direct targeting/stimulating of the DCs in vivo to accentuate their anti-cancer phenotype; stimulation of the DCs ex vivo and infusing them back into the host for carrying out anti-cancer effector function. Sipuleucel-T is the first DCs- based cancer vaccine, approved by the US Food and Drug Administration (FDA) for men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (CRPC).
Vaccine usually refers to biological products made from pathogenic microorganisms providing active acquired immunity to a particular disease. A vaccine typically contains an agent that resembles a disease-causing microorganism and is often made from weakened or killed forms of the microbe, its toxins, or one of its surface proteins. With the deeper understanding of the immune system and advances in biotechnology, scientists discovered a novel form of vaccines - cell vaccines, which are referred to dead or living cells that are used to induce an immune response in the recipient, with the aim of protecting them from future disease or infection. Compared to non-immune cells, immune cells are better choice in the production of cell vaccines for their significant roles in the adaptive immune responses. T-cell vaccination is immunization with inactivated autoreactive T cells (T cell vaccine, TCV). The concept of T-cell vaccination is, at least partially, analogous to classical vaccination against infectious disease. However, the agents to be eliminated or neutralized are not foreign microbial agents but a pathogenic autoreactive T-cell population. Research on T-cell vaccination so far has focused mostly on multiple sclerosis and to a lesser extent on rheumatoid arthritis, Crohn's disease and AIDS. Another immune cells that are widely studied for vaccination are dendritic cells (DCs). DC vaccine has been introduced as a new therapeutic strategy in cancer patients. DC-based immunotherapy is safe and can promote antitumor immune responses and prolong survival of cancer patients. DC-based immunotherapy approach can be employed in a couple of ways: direct targeting/stimulating of the DCs in vivo to accentuate their anti-cancer phenotype; stimulation of the DCs ex vivo and infusing them back into the host for carrying out anti-cancer effector function. Sipuleucel-T is the first DCs- based cancer vaccine, approved by the US Food and Drug Administration (FDA) for men with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer (CRPC).
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION