Exosome secretion levels are correlated with tumor stage and metastasis in the tumor microenvironment (TME). As signal molecules, exosomes contain selective repertoires of nucleic acids, proteins, lipids, and a series of metabolites. After their release into the extracellular environment, exosomes are taken up by recipient cells, both neighboring and distant, where they perform their regulatory functions. There is emerging evidence that tumor-derived exosomes (TEX) contribute to the recruitment and reprogramming of constituents associated with TME. Other cells in the TME also secrete exosomes. The two-way communication between tumor cells and the stroma or tumor can significantly affect the progression of disease and sensitivity of the tumor to treatment. Targeting exosomes could be a promising strategy in reshaping TME.
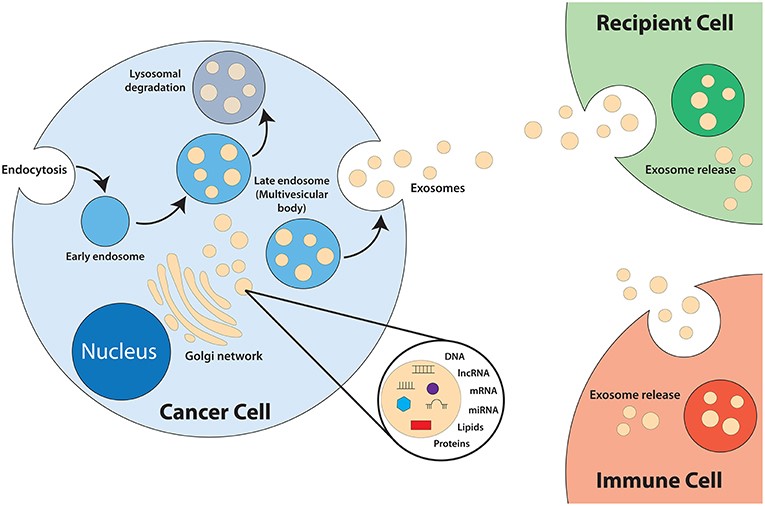 Fig.1 Biogenesis of exosomes.1,4
Fig.1 Biogenesis of exosomes.1,4
Almost all cells can secrete exosomes. Exosomes derived from different cells exhibit different characteristics and functions. Cells in TME including cancer-associated fibroblasts (CAFs), cancer stem cells (CSCs), mesenchymal stromal cells (MSCs), tumor microenvironmental immune cells (TMICs), etc. use exosomes to transfer messages, contributing to tumor growth, epithelial-mesenchymal transition (EMT), tumor metastasis and drug resistance through a variety of mechanisms.
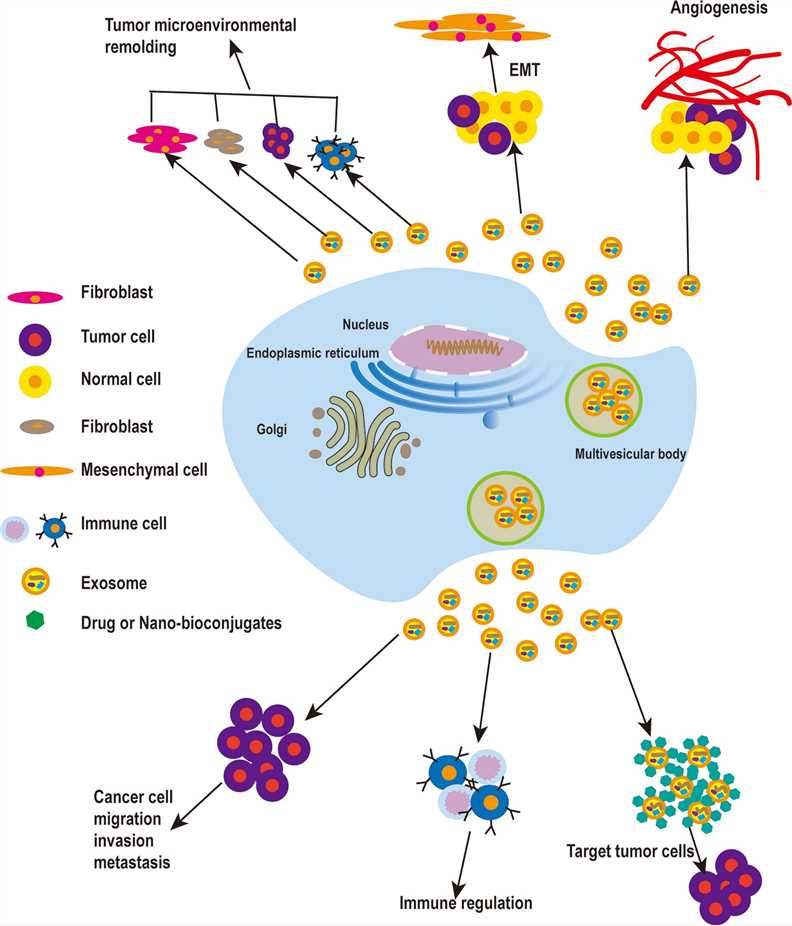 Fig.2 The roles of exosomes in tumors.2,4
Fig.2 The roles of exosomes in tumors.2,4
TEX can deliver multiple signals to surrounding cells and lay a pivotal role in tumor growth, angiogenesis and metastasis. TEX can
Exosomes derived from nontumor cells also can influence the TME.
Exosomes, and the genetic material and proteins that they contain, have shown promise as useful indicators of tumor burden, and prognosis, and perhaps even as a therapeutic treatment, as they modulate so many aspects of heterotypic cell-cell interaction in the TME. Currently, several different methods have been developed for tumor therapies: (1) using naturally-derived exosomes from immune cells to suppress tumor cells; (2) inhibiting the release of tumor; (3) using exosomes as gene carriers; and (4) using exosomes as anti-tumor drug carriers; 5) exosomes derived from specific sites in the body can be promising candidates for anti-tumor vaccines since they can present antigens against that specific type of tumors.
Exosomal drug delivery system with minimal toxicity, biocompatibility, tissue and tumor targeting, and long-circulating half-life emerges to be a superior choice. Therapeutic and targeted delivery at the tumor site can be achieved by modifying exosomes with corresponding targeting ligands. By engineering the parent cells to secrete modified exosomes, or alternatively, directly manipulating exosome content following secretion, exosomes are utilized for the therapeutic delivery of small molecules, proteins, and RNAs. Exosomes derived from specific sites in the body can be promising candidates for anti-tumor vaccines since they can present antigens against that specific type of tumor. DC-derived exosomes, containing MHC I, MHC II, CD86, and HSP70-90 chaperones, can trigger CD4+ and CD8+ T cell activation. TEXs express and transfer a wide spectrum of tumor-associated antigens to DCs that can prime tumor-specific cytotoxic T lymphocytes and induce potent antitumor immunity. Despite exerting remarkable vaccination effects, TEXs also display pro-tumorigenic characteristics as they resemble their original tumor cells. In particular, for the therapeutic application of exosomes, the source of exosomes needs to be carefully considered.
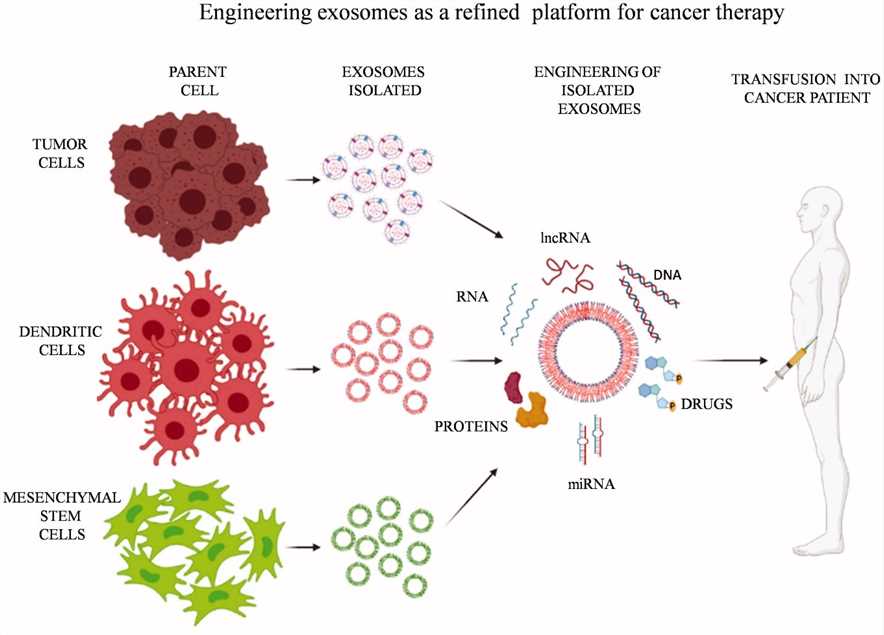 Fig.3 Manipulation of exosomes to carry next-generation chemotherapeutic drugs, modified DNA, RNA, peptides to the tumor cells.3,4
Fig.3 Manipulation of exosomes to carry next-generation chemotherapeutic drugs, modified DNA, RNA, peptides to the tumor cells.3,4
Creative Biolabs is dedicated to offering comprehensive exosome-related products and services including drug loading into exosomes and exosome genetic modification. Please feel free to contact us if you are interested in our services.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION