At Creative Biolabs, we offer various therapy strategies to manage the cytokine release syndrome (CRS) which is caused after the administration of CAR-modified T cells. For genetic engineering approaches, we provide suicide gene therapy solutions, targeted activation solutions and other customized solutions for our worldwide clients.
As one of the most promising cancer treatment approaches, CAR-T cell therapy has made remarkable achievements in eliminating hematologic cancers. CARs consist of extracellular antigen binding moiety (scFv) and intracellular signaling domains. Upon antigen recognition, CAR-T cells will be activated and produce cytokines, resulting in anti-tumor immune responses and elimination of tumor cells. Even though CAR-T cell therapy is promising, many toxicities were observed in clinical responses. Some toxicities may to fatal to patients. One of the severe toxicities is the production of a huge quantity of cytokines. Hence, control the cytokine production is critical to ensure the clinical safety of gene-modified CAR-T cells.
To improve the safety of CAR-T cell therapy, scientists in Creative Biolabs have made many efforts to explore different strategies. With years of discovery, we have gained more experience in improving CAR-T cell therapy with a wide range of approaches. One of the most promising strategies is targeted activation of CAR-T cells in specific conditions, instead of being activated all the time. Here are several strategies for your reference. If you want to explore other strategies and need our help, please reach out to our scientists for assistance. We have a series of platforms to support all stages of your drug discovery and development programs prior to the IND stage.
This strategy is based on the principle that T cell activation can be controlled by antigen targeting activation with two different signals. Dual CARs are designed to target two tumor-associated antigens (TAA). This is to ensure the CAR-T cells will be activated only two TAAs are expressed on tumor cells.
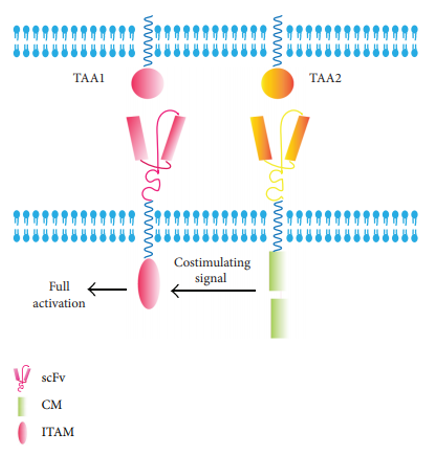 Fig.1 Dual-CAR.1
Fig.1 Dual-CAR.1
TanCAR is designed similarly to Dual-CAR. Two scFvs are tandemly arranged with the intracellular signaling domains. The CAR-T cell activation only occurs when two TAAs are expressed on target tumor cells.
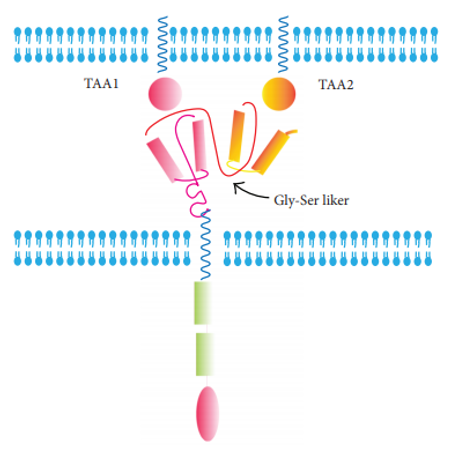 Fig.2 TanCAR.1
Fig.2 TanCAR.1
This strategy is designed based on the principle that the CAR-T cells will not be activated when a normal antigen is expressed on tumor cells. Upon recognition with the normal antigen, an inhibitory signal will be transduced and TAA-specific CAR will not be activated.
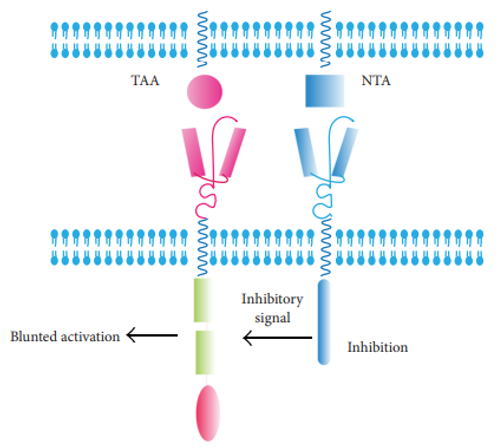 Fig.3 CAR+iCAR.1
Fig.3 CAR+iCAR.1
Synthetic Notch (synNotch) CAR is designed based on a Notch core and an artificial transcription factor. Upon TAA1 recognition, the transcription factor will be cleaved which primes the second CAR expression that is specific for TAA2.
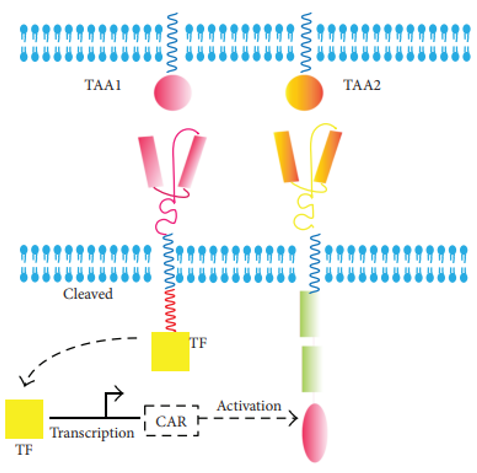 Fig.4 synNotch CAR.1
Fig.4 synNotch CAR.1
This approach is to distribute the CAR into two parts. The extracellular domain specific for TAA is expressed separately from the intracellular signaling transduction domain. The CAR-T cell is activated only when a heterodimerizing small molecule is administrated, resulting in the reassembling of two CAR components.
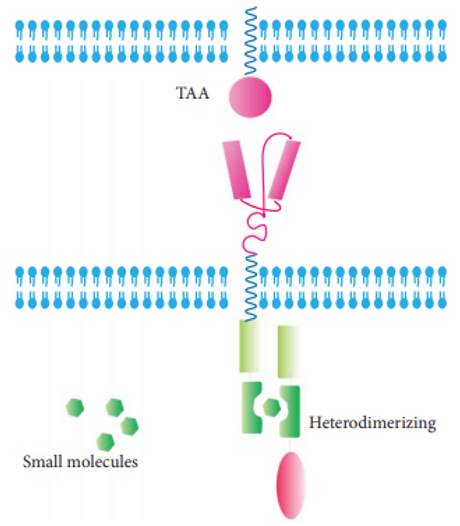 Fig.5 On-switch CAR.1
Fig.5 On-switch CAR.1
For this strategy, antibody is used for switching to control the activation of CAR-T cells. A TAA-specific Fab fragment is engrafted with a peptide neo-epitope (PNE). PNE will bind only PNE-specific switchable CAR-T cells.
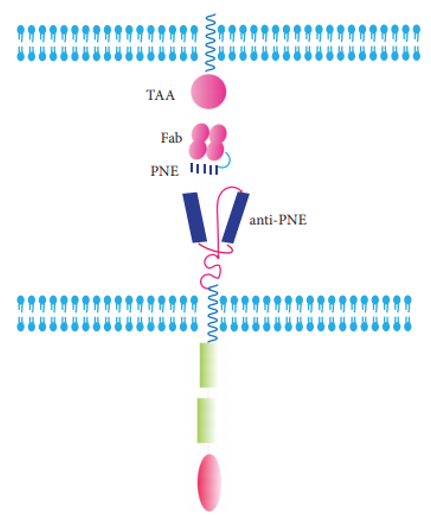 Fig.6 Antibody-based CAR.1
Fig.6 Antibody-based CAR.1
Creative Biolabs aims to bring more improved CAR-T cell therapies to our clients. Being the most trusted and reliable solution provider, we are confident to offer the most comprehensive services to support your drug discovery and development program. Our entire team is dedicated to providing exceptional scientific expertise via a series of services that exceed your expectations. Please contact us to learn how we can be involved in your program.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION