As an experienced contract research organization (CRO), Creative Biolabs now is dedicated to providing translational solutions to facilitate drug discovery and development services using world-renowned expertise in oncology, immunotherapy, drug discovery and pharmacokinetics/pharmacodynamics, etc. We strive to provide our customers with superior quality data to ensure the rapid and successful progression of their drug discovery programs for CRS management caused by chimeric antigen receptor (CAR)-T therapy.
CAR-T cell therapy has achieved impressive success in cancer patients, while along with the significant clinical benefits, some toxicities following the infusion of CAR-T cells have been reported (see Fig.1). The toxicities include cytokine release syndrome (CRS), anaphylaxis/allergy, neurological toxicity, "on-target, off-tumor" toxicity, etc. To date, the most prevalent adverse effect of CAR-T therapy is the onset of CRS that is a systemic inflammatory response following the activation of CAR-T cells. To control the CRS level that is harmful to patients, various strategies have been used to mitigate the symptoms of CRS without suppressing the antitumor efficacy of CAR-T cells. The current popular approaches to manage the CRS include corticosteroid and IL-6 receptor (IL-6R) blockade. However, prolonged use of high-dose corticosteroids can result in the ablation of the efficacy of CAR-T cell therapy. For IL-6R blockade, more studies are needed to investigate the negative effects of CAR-T cell persistence, proliferation, and anti-tumor efficacy. Hence, more strategies need to be explored to control CRS.
As immunotherapies become popular in clinical cancer treatment, a balance between efficacy achievement and toxicity management must be achieved. To prevent CRS and relevant neurotoxicity, various CAR-T cell genetic engineering strategies had been put forward, such as inhibitory CAR (iCAR), Tet-On System CARs, Switch CARs, inducible caspase CARs as well as CAR logic gates with built-in safety features. Creative Biolabs provides drug discovery and development services of different CAR-modified T cells to overcome the unwanted toxicities. With advanced CellRapeutics™ platform, our scientists offer unparalleled expertise and provide the customized genetic engineering solutions that facilitate your drug discovery program. With a growing and experienced multidisciplinary team of oncology, immunology, drug discovery, CAR-T cell therapy, we are committed to helping fast-forwarding delivery of more safety CAR-modified T cell therapy.
We offer a variety of suicide gene therapy to manage unexpected toxicities or eliminate transduced T cells. Suicide gene therapy is to incorporate a suicide gene to allow the selective death of CAR-T cells upon the administration of a safe prodrug. Our services include HSV-tk, iCasp9/AP1903 and targetable surface antigen such as CD20, truncated EGFR.
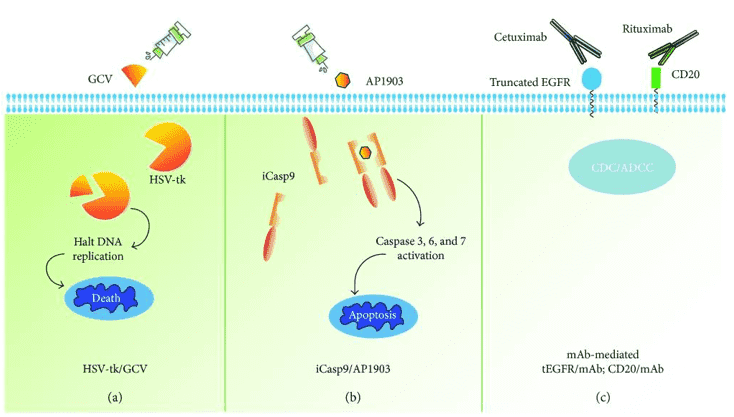 Fig.1 Approaches of overcoming toxicities by the suicide gene co-expression in T cells.1
Fig.1 Approaches of overcoming toxicities by the suicide gene co-expression in T cells.1
To enable the precise regulation of CAR-T cell therapy location, duration, and intensity, we offer targeted activation solutions including targeting two tumor-associated antigens and switch-mediated activation. For targeting two TAA strategies, we have dual-CAR, TanCAR, iCAR, synNotch CAR. For switch-mediated activation, we offer on-switch CAR and recombinant antibody-based CAR. We are dedicated to a variety of engineered CARs to facilitate your programs.
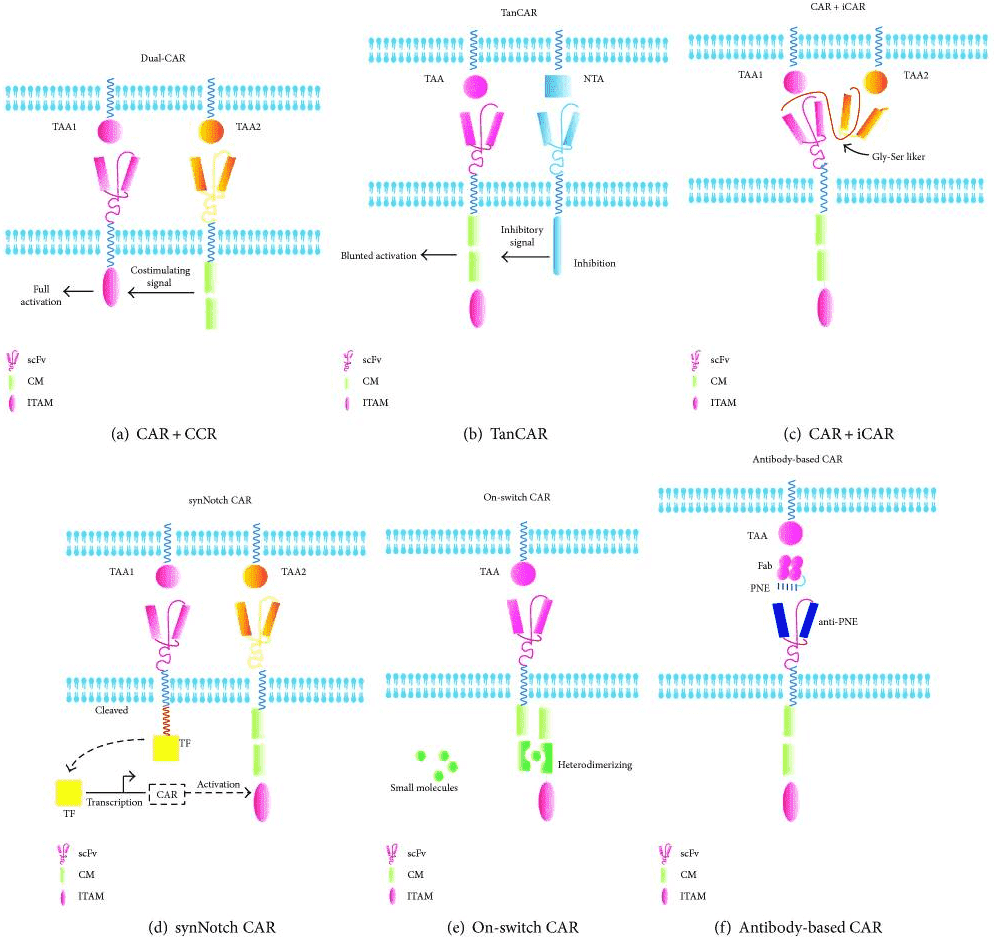 Fig.2 Targeted activation strategies for T cells to overcome toxicities.1
Fig.2 Targeted activation strategies for T cells to overcome toxicities.1
In addition to the approaches mentioned above, we also provide other solutions to tune down the toxicities of CAR-T cells by controlling the expression time or modulating the affinity of CARs. For any further solutions, please feel free to reach out to our scientists for further assistance.
As a flexible partner in the complex process of drug discovery and development for CRS management caused by immunotherapies, Creative Biolabs aspires to become the trusted solution provider of choice for all CRS management drug discovery and development. We are committed to increasing the probability of success and reducing the overall development timeline for our clients. Please don't hesitate to contact us to know more.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION