Cancer-associated fibroblasts (CAFs) are a unique cell population of the tumor microenvironment (TME) and play an important contributory role in the TME. CAFs are recognized as a heterogeneous cell type in TME and originate from various cells. They can originate from normal tissue-resident fibroblasts, as well as a number of non-fibroblastic mesenchymal cells or even tumor cells (Fig.1). As a source of growth factors, cytokines, chemokines, and other regulatory molecules, they participate in cancer progression, metastasis, angiogenesis, immune cell reprogramming, and therapeutic resistance.
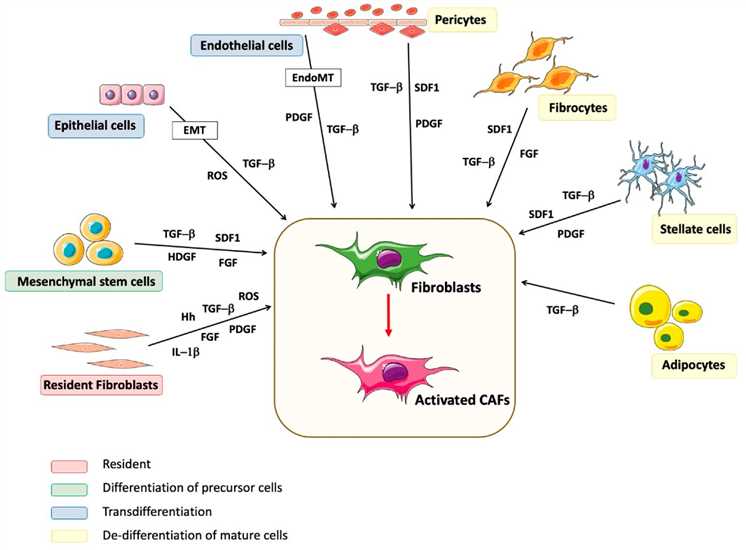 Fig.1 Origin of CAFs.1,3
Fig.1 Origin of CAFs.1,3
Fibroblasts are one of the abundant factors in TME which have a major impact on tumor behavior. Although normal fibroblasts demonstrate anti-tumor effects, CAFs are principal participants in tumor growth and invasion. Cancer cells can drive fibroblast activation. In addition to malignant cells, endothelial, inflammatory, and immune cells in the TME also secrete mediators of fibroblast activation. Moreover, activated fibroblasts produce factors and matrix that drive their autocrine activation in a feed-forward manner, as fibroblasts are responsive to substratum composition and stiffness.
CAFs are a substantial source of matrix production, growth factors, cytokines, and exosomes. CAFs produce large amounts of ECM proteins creating a favorable stromal environment. Soluble factors can contribute to changes in tumor growth, invasion, and the immune microenvironment, which is also affected by the altered metabolic state of the tumor. The secretome of CAFs also influences other components of the TME, including macrophages, T cells, and tumor cells. Moreover, the exchange of metabolites between cancer cells and CAFs is an additional avenue to support tumor growth.
Summary of CAFs Functions
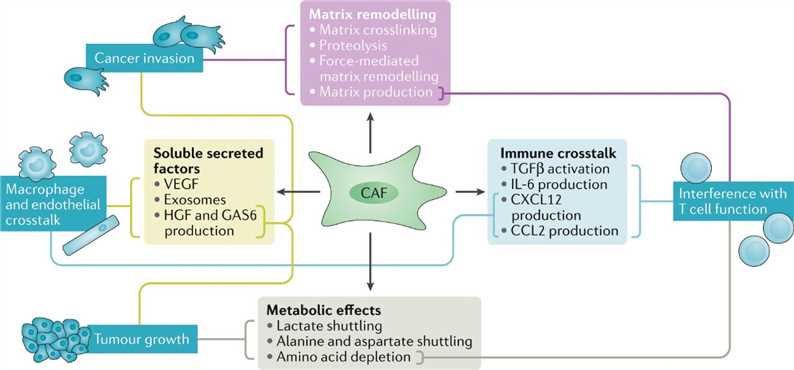 Fig.2 Summary of CAF functions and the mechanisms by which they are achieved.2,3
Fig.2 Summary of CAF functions and the mechanisms by which they are achieved.2,3
Drugs that target CAFs have emerged as a critical complement to immunotherapies in multiple solid tumors. There are many strategies under investigation for targeting CAFs including depletion, blocking their tumor-promoting functions, inhibiting their activation, and skewing them to a normal or even tumor-suppressive phenotype.
Fibroblast activation protein (FAP) has emerged over the last decade as the most promising immunotherapeutic target. Targeting FAP attenuates activated tumor-promoting traits in CAFs and augments immunotherapy treatments. There are many strategies to deplete CAFs including CAR-T therapy, monoclonal antibody, oncolytic viruses, etc.
NADPH oxidase 4 (NOX4) is one of the target genes of TGF-β signaling in fibroblasts. NOX4 inhibition can prevent and reverse CAFs activation, and reduce ECM deposition and CAF-mediated tumor cell invasion and migration. TGF-β blockade and angiotensin receptor inhibition (e.g. Vitamin D) are alternative approaches to inhibit CAFs activation.
Targeting downstream effectors of CAFs, mainly CAF-derived cytokines and chemokines, can interfere with communication between cancer cells and stromal cells including CAFs. While inhabiting a single pathway may exert some efficacy, it is unlikely to eradicate tumors.
ECM modifications play an important role in tumor progression (survival, angiogenesis, pre-metastatic niche, chemoresistance, etc). Therefore, targeting ECM components holds much promise for improved cancer management. ECM and associated signaling can be targeted to induce stromal depletion and increase immune T cell infiltration.
Creative Biolabs is committed to providing flexible services and products to support every aspect of our clients' needs. Now we are dedicated to developing strategies to reshape TME. CAFs are important components in TME and attract increasing attention as a therapeutic target in cancer. Various CAF marker antibodies at our lab are available for CAF validation. Custom services include CAR-T, Oncolytic Virus, antibody development services, etc. In addition, we offer expertise to help accelerate the process of targeting CAFs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION