Hypoxia in locally advanced solid tumors develops due to high cell proliferation rates, increased oxygen consumption and aberrant blood vessel development. Hypoxia is an important feature of the tumor microenvironment (TME) and is closely associated with cell proliferation, angiogenesis, metabolism and the tumor immune response. While hypoxia has been strongly linked to tumor metastasis and poor clinical outcome for patients, it seems to actually have a dual role: insufficient oxygen limits tumor cell division while at the same time selecting for more malignant cells and inducing cell adaptations allowing for more invasive behavior.
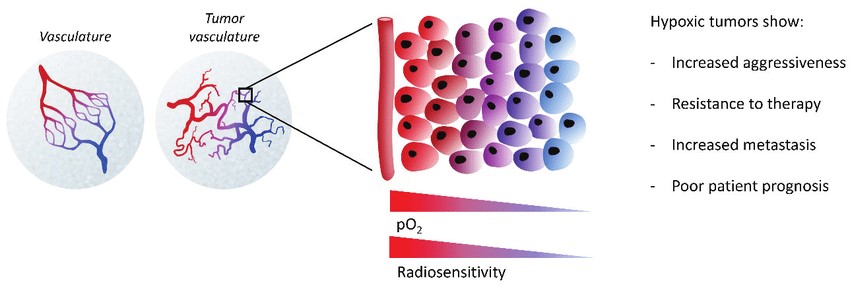 Fig.1 TME and hypoxia.1,3
Fig.1 TME and hypoxia.1,3
The hypoxic response is mainly ascribed to hypoxia-inducible factors (HIFs). The hypoxic TME exerts a far-reaching impact on diverse aspects of tumor biology.
Hypoxia, through HIF-1α, induces the secretion of pro-angiogenic factors including vascular endothelial growth factor (VEGF), angiopoietin 2 (Ang-2), and fibroblast growth factor, thus leading to a vicious circle. Abnormal tumor vascularization can impair blood flow, aggravate hypoxia, and limit the delivery of nutrients and therapeutics, including antibodies and immune cells.
In hypoxic conditions, cancer cell metabolism undergoes a shift from oxidative phosphorylation to aerobic glycolysis and the production of lactic acid, which results in increased tumor acidosis. Reprogramming glucose flux is considered as a major factor to shape the TME via increase of HIF-1α levels in rapidly growing tumor cells within hypoxic regions.
Hypoxic zones in solid tumors are infiltrated by a large number of immunosuppressive cells within the TME including myeloid-derived stromal cells (MDSCs), tumor-associated macrophages (TAMs), and T-regulatory (Treg) cells. In addition, hypoxia can suppress the anti-tumor activities of T cells by HIF and inhibit the stimulatory capacity of DCs for activating T cell functions.
HIF1 is involved in the regulation of immune checkpoints. Several important immune checkpoint molecules, including PD-L1, human leukocyte antigen G (HLA-G), cluster of differentiation 47 (CD47), and V-domain Ig suppressor of T-cell activation (VISTA), are regulated by hypoxia and contribute to immune evasion.
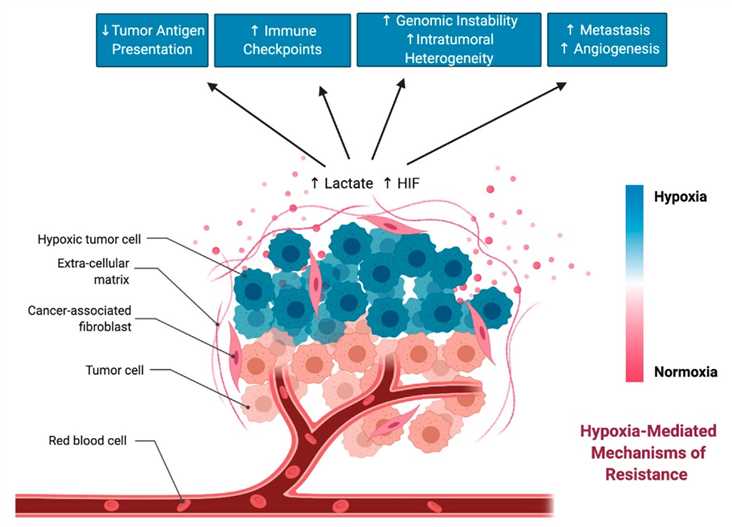 Fig.2 Hypoxia-mediated changes in the TME that can drive resistance to immunotherapy.2,3
Fig.2 Hypoxia-mediated changes in the TME that can drive resistance to immunotherapy.2,3
For a long time, hypoxia-relevant signaling pathways have been among the most attractive therapeutic targets in cancer drug development. One strategy for targeting hypoxia proposes the inhibition of HIFs, and their downstream targets or upstream signaling partners. Many HIF inhibitors have been developed but not approved due to bad efficacy in clinical trials. Due to the hypoxia-driven reprogramming metabolism, interfering with the main process of the metabolic switch is an effective anti-cancer strategy. In addition, the use of supplemental oxygen as a simple means to enhance immunotherapy is appealing.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION