Research Progress of Protein Glycosylation Modification
Overview of Protein Glycosylation Modification
Glycosylation is essential in all domains of life for diverse physiological functions, such as modulating protein stability, mediating cell adhesion, facilitating cell-cell communication, or evading recognition by other organisms. Protein glycosylation involves the covalent addition of glycans to the amino group of an asparagine (N-linked) or the hydroxy group of a serine or threonine (O-linked). In eukaryotes, glycosylation predominantly occurs in the endoplasmic reticulum and Golgi apparatus. Here, membrane-bound glycosyltransferases and glycosidases sequentially add or remove monosaccharides, thereby creating a growing glycan side chain of variable length and diverse types of branching. Specific glycosylation sites on a protein may or may not carry a glycan (referred to as microheterogeneity), and different copies of the same protein may carry different glycans on the same site (referred to as microheterogeneity). While this glycan heterogeneity may suggest that some functions of glycosylation do not require specific glycans, glycosylation is not a purely random process. In fact, at certain sites, glycoproteins are highly sensitive to their glycosylation, as subtle alterations in glycan structure can lead to protein malfunction, implying failed development, disease, or cancer. However, glycosylation occurs without a template, and the molecular mechanisms by which the cell achieves non-random glycan assembly remain poorly understood.
Cell-Based Protein Glycoengineering
-
Glycoengineering Based on Mammalian Cells
Correct glycan structures are crucial for potency and control of pharmacokinetic and pharmacodynamic properties of glycoprotein biologics and therapeutic carbohydrates. Protein glycosylation and synthesis of the glycosaminoglycan (GAG) portion of proteoglycans are nontemplated, and thus, significant heterogeneity can arise from organism to organism, cell type to cell type, and even between different culture conditions. Chinese hamster ovary (CHO) cells are the most widely used host for manufacturing complex biopharmaceuticals due to their ability to replicate folding and post-translational modifications, including glycosylation patterns, found in human proteins. In antibodies, the most widely used production cell lines are CHO, NS0, and Sp2/0, in various engineered forms. These cell lines produce IgG Fc glycoforms bearing the top eight oligosaccharides.
-
Glycoengineering Based on Non-mammalian Cells
Scientists have also chosen many different types of non-mammalian cells for protein glycoengineering, including plant, insect, yeast, and bacteria cells. Plants, as eukaryotic organisms, can be instilled with many of the desirable mammalian traits while maintaining their significant bioprocessing advantages. This facilitates the expression of complex human proteins (e.g., antibodies) with intricate post-translational modifications (e.g., N- and O-glycosylation). Insect protein glycosylation pathways are complex and tissue- and/or developmental stage-specific. The most obvious targets for insect expression system glycoengineering are the functions required for N- and O -glycan elongation. These include functions mediating the biosynthesis and transport of donor nucleotide glycans and the enzymatic transfer of glycans from those donors to relevant acceptor substrates. Yeasts are capable of performing many human posttranslational modification reactions, including N-linked glycosylation. However, the N-linked glycans from yeast differ significantly from those of mammalian cells and humans, which has compromised their therapeutic utility. The challenge for glycoengineering has been the elimination of the endogenous hyper-mannosylated yeast glycans, followed by the introduction of elements required to generate human-like sialylated complex glycans. Bacterial glycoengineering has experienced a rise in popularity following the discovery of bacterial N-glycosylation systems with the ability to generate non-native glycans and conjugate these to recombinantly expressed proteins.
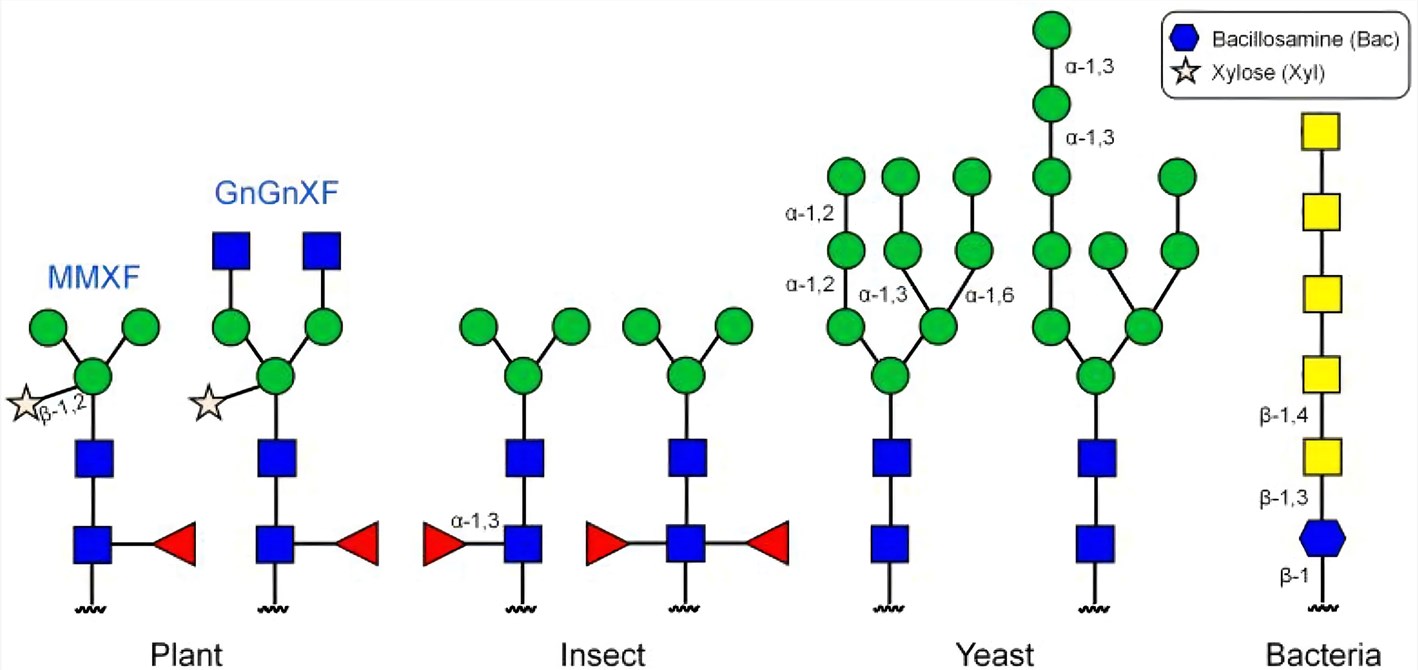 Fig. 1 N-linked glycans on glycoproteins produced in different expression systems.1, 2
Fig. 1 N-linked glycans on glycoproteins produced in different expression systems.1, 2
Chemistry-Based Protein Glycoengineering
-
Glycoengineering Based on Biochemistry
Protein glycoengineering based on biochemistry methods is mainly accomplished through the use of biochemical reactions catalyzed by a variety of glycosidases and glycosyltransferases. Glycosidases catalyze the cleavage of glycosidic bonds, while glycosyltransferases catalyze the opposite reaction, glycosidic bond formation, mainly using glycan nucleotides as glycosyl donors. Enzymatic synthesis is generally more convenient, although enzymes to construct specific glycan structures must still be identified, optimized, and purified. Moreover, using enzymes does limit the synthesis based on the sequence specificity and activity of the enzymes being used, and producing multiply glycosylated proteins with different glycans at each site is still difficult in almost all cases.
-
Glycoengineering Based on Organic Chemistry
Approaches that use chemical synthesis are supremely flexible since chemically introducing glycans does not depend on a protein’s sequence or local structure. The chemical synthesis also allows the construction of glycoproteins carrying different glycan structures at individual sites with relative ease. However, despite the amount of effort that has gone into optimizing synthetic procedures for glycoprotein synthesis, the process remains difficult and tedious in most cases.
Services at Creative Biolabs
Protein glycosylation modification has been entered the vision of glycoprotein researchers for many years, and its importance is self-evident. Creative Biolabs has developed a comprehensive technology platform to help with glycoprotein analysis services:
If you are interested in our services or you have any other requirements in glycoprotein services, please don't hesitate to contact us for more information.
References
-
Ma, Bo, et al. "Protein glycoengineering: an approach for improving protein properties." Frontiers in Chemistry 8 (2020): 622.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig. 1 N-linked glycans on glycoproteins produced in different expression systems.1, 2
Fig. 1 N-linked glycans on glycoproteins produced in different expression systems.1, 2

