Deglycosylation Strategy
Overview of Declycosylation
With the rapid development of proteomics and glycomics research, glycoproteins have become the focus of many pieces of research. Glycoproteins are binding proteins composed of oligosaccharide chains and proteins connected by covalent bonds. They are widely present in animals, plants, and microorganisms in the form of enzymes, lectins, immunoglobulins, and membrane proteins. Many pieces of literature reported that glycoprotein has anti-oxidation, anti-tumor, lowering blood glycan, enhancing immunity, anti-radiation, and other effects. The oligosaccharide chains in glycoproteins are linked to the protein through glycosylation in the process of co-translational modification or post-translational modification. According to the connection site of the glycan chain on the peptide chain, there are N-linked glycan chains, O-linked glycan chains, glycosylphosphatidylinositol-anchored protein (GPI), and C-linked glycan chains. Currently, the most studied are N- and O-linked glycoproteins. Glycoprotein is consists of glycan chains and peptide chains. Due to its huge and complex structure, methods to remove the glycan chain in glycoproteins are required. The methods include enzymatic methods, chemical methods, glycosylation inhibitors, recombinant DNA technology, and certain glycan. The removal of oligosaccharide chains from glycoproteins by mutant cells with defective basic steps is an important way to study the structure and function of glycoproteins. At present, the most commonly used deglycosylation methods are enzymatic and chemical methods.
Deglycosylation Strategies
The chemical method has a fast reaction rate and can achieve complete deglycosylation. The hydrazinolysis method is used to release GlcNAc-Asn-linked N-glycoprotein. The trifluoromethanesulfonic acid method has a rapid reaction, mild conditions, no effect on protein conformation, and is suitable for all types of glycan chain analysis. The periodate oxidation-β elimination method is widely used in O-glycoproteins containing GalNAc-Ser/Thr. The reaction conditions are mild, have little effect on peptide chains and the removal of O-glycans is relatively thorough, so it is the most ideal method of the chemical methods for deglycosylation. The trifluoroacetic acid method is suitable for neutral O-linked glycoproteins. Although chemical methods are fast to remove glycan bases, they also release harmful chemicals and destroy the structure of proteins and glycan chains. Chemical methods may cause undesirable side reactions, such as epimerization, hydration, and hydroxylation.
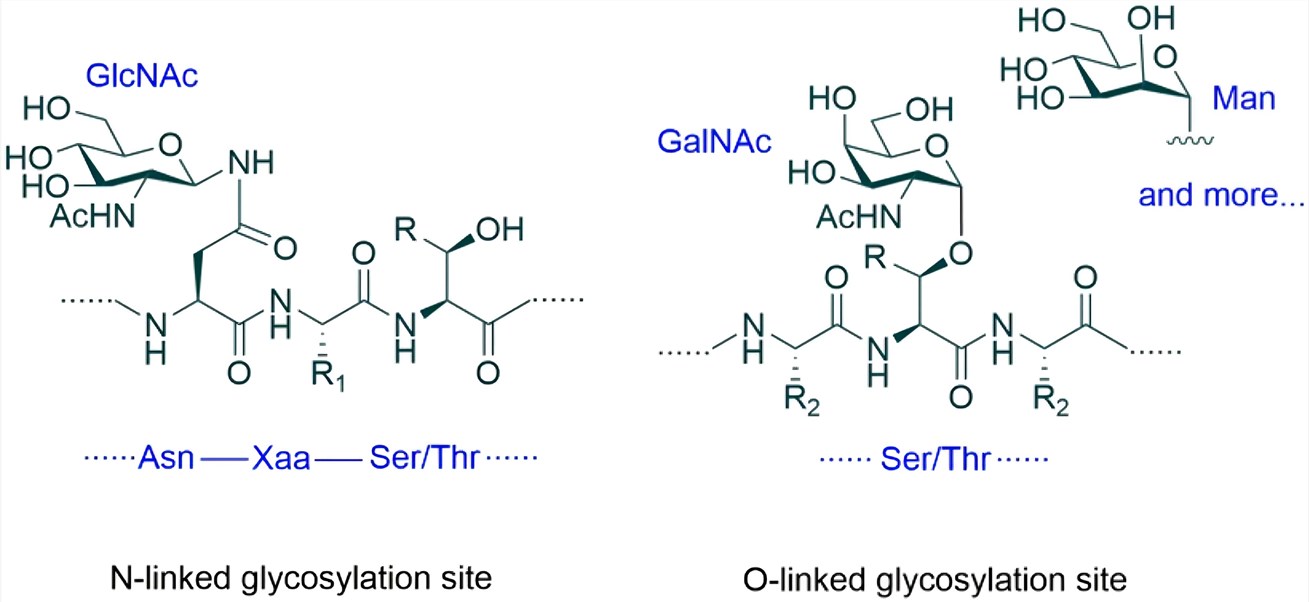 Fig.1 N-linked and O-linked protein glycosylation.1, 2
Fig.1 N-linked and O-linked protein glycosylation.1, 2
The enzymatic conditions are mild and do not affect the peptide structure. Enzymatic hydrolysis can be carried out in solution or in gel, which is very convenient for application. For N-linked glycopeptides, PNGase A enzymes and PNGase F enzymes have wide specificity for oligosaccharide structures and strong specificity for peptide molecules. PNGase F is the most widely used in enzymatic deglycosylation. It can tolerate a certain concentration of denaturants such as TX-100 and SDS. PNGase A enzyme can effectively remove seaweed glycoproteins, but it is not suitable for N-acetylglucosamine glycoproteins containing sialic acid groups. Endo H and Endo F are very useful in the analysis of glycan chain structure and the study of the relationship between structure and function because they cut a certain bond exclusively from the inside of the glycan chain. Both of them have weak specificity to proteins, and can only partially remove glycosyl groups, leaving GlcNAc residues on Asn after enzymolysis. For O-linked glycopeptides, a series of exoglycosidases are required to release oligosaccharides. Although the enzymatic method is mild, in the traditional enzymatic deglycosylation process, the deglycosylation process generally leads to irreversible denaturation of the target glycoprotein, and it takes a long time and usually requires an overnight reaction.
Services at Creative Biolabs
Deglycosylation is an important part of glycoprotein research to help with glycal modification. Creative Biolabs has developed a comprehensive glycoprotein research platform. We are pleased to provide global clients with high-quality glycoprotein analysis services:
If you are interested in our services or you need any help in glycoprotein research, please don't hesitate to contact us for more information.
References
-
Ma, Bo, et al. "Protein glycoengineering: an approach for improving protein properties." Frontiers in Chemistry 8 (2020): 622.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 N-linked and O-linked protein glycosylation.1, 2
Fig.1 N-linked and O-linked protein glycosylation.1, 2

