Motor neurons are critical components of the central nervous system (CNS), responsible for conveying electrical signals from the spinal cord to skeletal muscles, thus facilitating voluntary movement. The ability to generate motor neurons from induced pluripotent stem cells (iPSCs) offers transformative potential for disease modeling, drug discovery, neurotoxicity assessment, and potential regenerative therapies.
Creative Biolabs provides a robust, scalable, and highly reproducible protocol to generate high-purity, functional motor neurons from human iPSCs. This protocol outlines each critical step — from iPSC maintenance to terminal motor neuron maturation — and integrates quality control measures to ensure cellular identity, viability, and functionality.
iPSCs are capable of differentiating into any cell type, including spinal motor neurons. This approach allows for the derivation of patient-specific motor neurons that carry the individual's genetic background, thereby facilitating highly personalized disease modeling and drug response testing.
The differentiation of iPSCs into motor neurons typically mimics key steps of in vivo neurodevelopment. This multistep process includes:
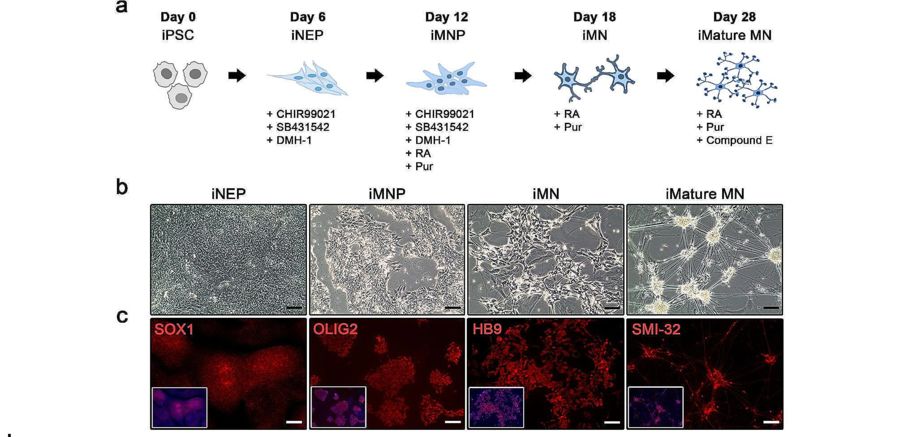 Fig.1 Generation of iPSC-derived motor neurons using small molecules in vitro.1,2
Fig.1 Generation of iPSC-derived motor neurons using small molecules in vitro.1,2
At Creative Biolabs, we have fine-tuned this process into a robust and modular platform that consistently delivers high yields of physiologically relevant motor neurons.
By leveraging our expertise in iPSC biology, developmental neuroscience, and cellular engineering, Creative Biolabs is committed to empowering researchers with reliable access to functional, disease-relevant motor neurons that accelerate innovation across neuroscience and regenerative medicine.
| Reagent | Purpose |
|---|---|
| Matrigel or vitronectin | Coating substrate |
| Essential 8 Medium | iPSC maintenance |
| Accutase | Gentle cell dissociation |
| DMEM/F12 + N2 + B27 | Basal differentiation media |
| Retinoic acid (RA) | Caudalization |
| Smoothened Agonist (SAG) | Ventralization |
| BDNF, GDNF, CNTF | Neurotrophic factors |
| Laminin | Final coating for maturation |
Coat plates with Matrigel or vitronectin. Seed iPSCs onto coated plates in Essential Medium. Feed daily and monitor morphology. Colonies should be compact with defined borders. Passage using Accutase every 3–5 days when colonies reach ~70–80% confluency. Ensure cells are free from spontaneous differentiation before initiating differentiation.

Replace medium with neural induction medium (DMEM/F12 + N2 + B27) supplemented with: SB431542, LDN193189, CHIR99021. Incubate for 6 days, changing medium daily. Neuroepithelial structures begin forming, cells become rosette-like.

Switch to patterning medium. Add RA for caudalization. Add SAG for ventralization. Supplement medium with: CHIR99021 to fine-tune Wnt signaling. Continue daily medium change for 5–6 days. Cells will exhibit a pseudostratified morphology characteristic of ventral spinal cord progenitors.

Change to motor neuron maturation medium, consisting of Neurobasal + B27, supplemented with: BDNF, GDNF, CNTF, DAPT, Ascorbic acid. Plate cells on laminin-coated plates to enhance attachment and maturation. Maintain cultures for at least 3 weeks with medium change every 2–3 days.
We employ a rigorous immunocytochemical and transcriptional profiling panel to verify the developmental trajectory and terminal fate of differentiated cells
| Differentiation Stage | Key Markers | Detection Methods |
|---|---|---|
| Neural progenitors | SOX1, PAX6 | IF, qPCR |
| Spinal identity | HOXB4, HOXC6 | IF, qPCR |
| Motor neuron progenitors | OLIG2, NKX6.1 | IF, flow cytometry |
| Post-mitotic motor neurons | HB9, ISL1, ChAT, TUJ1, SMI-32 | IF, qPCR, Western blot |
| Mature functionality | Synaptophysin, VAChT | IF, qPCR, ICC |
The process of differentiating iPSCs into high-purity, functional motor neurons is complex and highly sensitive to variations in reagents, cell line quality, and culture conditions. At Creative Biolabs, we have compiled a detailed troubleshooting guide to help clients navigate common technical challenges and optimize each stage of the workflow.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low efficiency of neural induction |
|
|
| Cell death during patterning or ventralization |
|
|
| Low yield of motor neurons |
|
|
| Poor neurite outgrowth or cell attachment during maturation |
|
|
| Heterogeneous cell population with non-motor neuron lineages |
|
|
Creative Biolabs also provides custom consultation for clients who face persistent issues during iPSC differentiation workflows. Our team can perform remote assessments, optimize protocols based on specific cell lines, and even execute partial or full-service differentiation on behalf of your project.
Creative Biolabs offers a comprehensive suite of stem cell differentiation and neuronal characterization services.
Tailored protocols for neuronal, cardiac, hepatic, and glial lineages.
Generation of integration-free iPSC lines from somatic tissues.
CRISPR/Cas9 knock-in/knock-out strategies for isogenic controls.
We tailor our solutions based on client cell lines, disease models, or compound testing needs.
References
Created July 2025
For Research Use Only. Not For Clinical Use.