Smooth muscle cells (SMCs) play an essential role in vascular homeostasis, organ function, and tissue architecture. Derived from mesodermal origin, SMCs are found in the walls of blood vessels, gastrointestinal tract, airways, and the genitourinary system. Dysregulation of SMC function is implicated in various diseases including atherosclerosis, hypertension, asthma, and bladder dysfunction.
Induced pluripotent stem cells (iPSCs) provide a platform for generating patient-specific SMCs for disease modeling, drug discovery, regenerative medicine, and vascular tissue engineering. Creative Biolabs offers an optimized, reproducible protocol to derive functional SMCs from iPSCs under defined, xeno-free conditions.
The derivation of SMCs from iPSCs is a multi-stage process that recapitulates early embryogenesis, particularly the mesodermal lineage trajectory. SMCs originate from the mesoderm germ layer, with some regional variation arising from neural crest or lateral plate mesoderm depending on the anatomical site. This biological complexity is mirrored in vitro, necessitating precise temporal control of signaling pathways to guide lineage specification and maturation.
The successful differentiation of iPSCs into SMCs relies on the dynamic interplay of several signaling pathways.
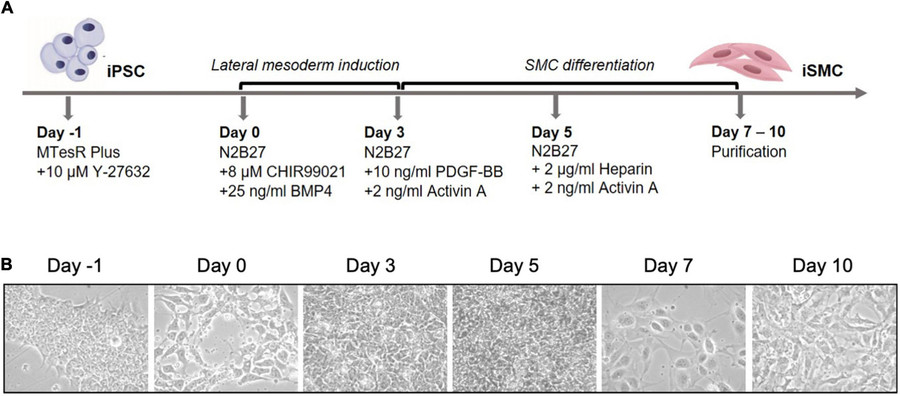 Fig.1 Differentiation of iPSCs into iSMCs.1,2
Fig.1 Differentiation of iPSCs into iSMCs.1,2
There are two major approaches to generating iPSC-derived SMCs:
At Creative Biolabs, our proprietary directed differentiation protocol ensures highly consistent SMC yield under xeno-free, defined culture conditions.
| Component | Details |
|---|---|
| iPSCs | Feeder-free, validated |
| Matrigel or Vitronectin | Substrate coating |
| RPMI 1640 | Basal medium |
| B27 supplement (minus insulin) | Differentiation supplement |
| BMP4, Activin A | Mesoderm induction |
| VEGF, bFGF | Vascular progenitor support |
| PDGF-BB, TGF-β1 | SMC differentiation and maturation |
| Antibodies (α-SMA, CNN1, MYH11) | For flow cytometry or immunofluorescence |
| qPCR reagents | For gene expression analysis |
Culture iPSCs in feeder-free conditions on Matrigel- or vitronectin-coated plates using Essential 8 medium. Passage cells at ~70-80% confluence using EDTA. Ensure pluripotency marker expression (OCT4, SOX2, NANOG).

Replace medium with RPMI 1640 supplemented with B27 (minus insulin), BMP4, and Activin A. Incubate for 48 hours, monitoring morphology changes (elongated mesodermal-like cells).

Replace medium with RPMI + B27 (minus insulin) + VEGF + bFGF. Culture for another 48–72 hours, allowing emergence of CD34+/KDR+ vascular progenitors.

Switch to RPMI + B27 (complete) containing PDGF-BB and TGF-β1. Change medium every other day. Monitor expression of early SMC markers like α-SMA from day 8 onwards.

Continue culture in SMC medium supplemented with PDGF-BB and TGF-β1. Expand matured cells on collagen-coated plates. Validate expression of mature markers such as calponin and MYH11 via qPCR or IF.
Robust quality control is essential for verifying the identity, purity, and functionality of iPSC-derived SMCs. At Creative Biolabs, we implement the following assays:
Below is a comprehensive troubleshooting guide and a set of actionable optimization tips to help maximize reproducibility and performance.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low mesoderm induction efficiency | Inactive BMP4/Activin A, over-confluent iPSCs |
|
| Poor survival post-induction | Cytokine stress, lack of survival factors |
|
| Heterogeneous vascular progenitor population | Non-uniform mesoderm induction, variable VEGF response |
|
| Weak expression of SMC markers (α-SMA, CNN1) | Insufficient PDGF-BB/TGF-β1 exposure or incorrect timing |
|
| Loss of contractility phenotype | Overpassaging, prolonged culture in growth-promoting media |
|
| Excessive cell detachment | Poor matrix coating, mechanical stress during handling |
|
| Batch-to-batch variation | Growth factor inconsistency, reagent degradation |
|
Optimization Tips for Improved Efficiency and Reproducibility
Creative Biolabs offers an extensive portfolio of iPSC-based vascular research solutions and customized cell differentiation services.
Generating smooth muscle cells from iPSCs is a powerful tool to bridge fundamental research with translational medicine. With a carefully optimized protocol and quality-controlled workflow, Creative Biolabs enables researchers to harness the full potential of iPSC-derived SMCs for disease modeling, pharmacological testing, and tissue engineering. Contact us today to initiate a customized SMC differentiation project tailored to your research needs.
References
For Research Use Only. Not For Clinical Use.