Advances in induced pluripotent stem cell (iPSC) technology, which generates patient-specific tendon-like cells, offer a powerful solution. At Creative Biolabs, we focus on directing iPSC through precise differentiation protocols to obtain functional tendon-like cells for applications in tissue engineering, regenerative therapies, and disease modeling.
This protocol outlines a step-by-step approach to differentiate from iPSC to tendon-like cells, integrating developmental biology insights, molecular signaling control, and scalable production capabilities.
The differentiation of tendon-like cells from iPSCs is a highly orchestrated, multistage process that mirrors embryonic tendon development. Tendon formation, or tenogenesis, originates from the paraxial mesoderm and proceeds through sequential specification into somites, sclerotome, syndetome, and eventually mature tenocytes. This process is governed by a finely balanced interplay of biochemical signaling cues and biomechanical stimuli.
During embryogenesis, tendons are derived from the syndetome, a specialized subdomain of the sclerotome, which in turn arises from paraxial mesoderm. The early commitment to mesoderm is driven by Wnt and BMP signaling, followed by sclerotome patterning orchestrated by Sonic Hedgehog (Shh) emanating from the notochord and floor plate. The critical tenogenic transcription factor Scleraxis (SCX) is activated in response to FGF8, TGF-β, and GDF5, which collectively promote tendon progenitor identity and matrix gene expression.
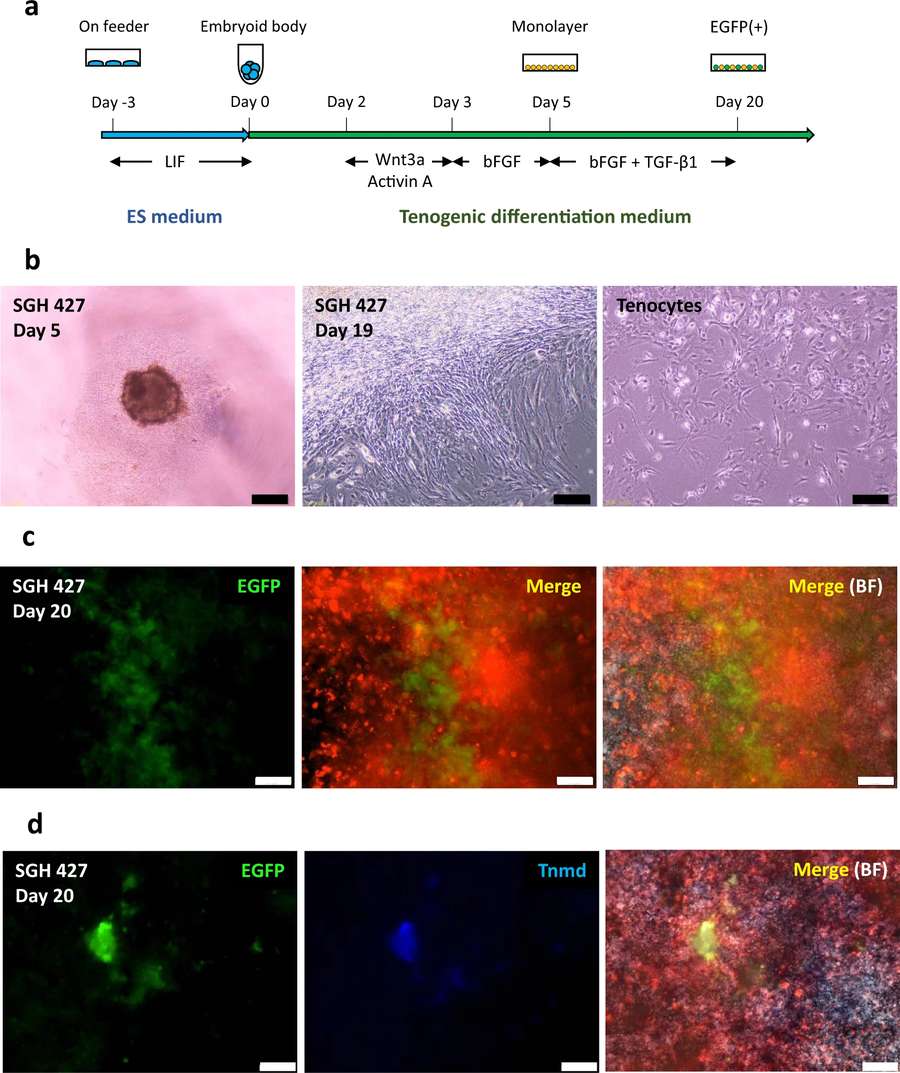 Fig.1 Induction of tenocyte-like cells from iPSCs.1,2
Fig.1 Induction of tenocyte-like cells from iPSCs.1,2
Tendon-like cells are defined by a unique set of markers:
Monitoring the sequential expression of these markers ensures the fidelity of differentiation and the functional relevance of the resulting cells.
| Component | Details |
|---|---|
| iPSC culture medium | mTeSR™1 or equivalent |
| Mesoderm induction media | Containing CHIR99021, BMP4 |
| Sclerotome induction media | Containing Shh agonist (SAG), FGF8 |
| Syndetome induction factors | TGF-β3, GDF5, FGF2 |
| ECM coating | Fibronectin, Collagen I |
| 3D scaffold (optional) | Hydrogel or decellularized tendon matrix |
Culture iPSCs on Matrigel-coated plates using mTeSR™1 medium. Passage when 70–80% confluent using EDTA. Ensure colonies are undifferentiated.

Replace medium with mesoderm induction medium: CHIR99021, BMP4. Incubate for 72 hours, changing medium daily. Confirm mesoderm markers: Brachyury (T), MESP1 expression by qPCR or immunofluorescence.

Treat with SAG, FGF8, BMP. Maintain for 2–3 days and confirm PAX1 and NKX3.2 expression.

Medium containing: TGF-β3, GDF5, FGF2. Cultivate in 2D or transition to 3D scaffold. Confirm expression of SCX, TNMD, COL1A1, and COL3A1 by qPCR and ICC.
Functional assays may include:
| Marker | Method | Stage |
|---|---|---|
| Brachyury (T), MESP1 | qPCR, ICC | Mesoderm |
| PAX1, NKX3.2 | qPCR | Sclerotome |
| SCX, TNMD | Immunostaining, Flow cytometry | Tendon progenitor |
| COL1A1, COL3A1 | ELISA, Western blot | Matrix production |
Efficient differentiation of tendon-like cells from iPSCs requires precise control over signaling pathways, timing, and culture conditions. Variability in response across cell lines and batches can lead to inconsistent outcomes. This section outlines common issues encountered during the process and provides practical solutions and advanced optimization strategies.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low mesodermal induction efficiency | Ineffective Wnt activation or BMP signaling |
|
| Heterogeneous cell populations after sclerotome stage | Incomplete BMP inhibition or timing error |
|
| Weak SCX/TNMD expression | Suboptimal TGF-β3 or FGF8 exposure |
|
| High cell death in 3D culture | Scaffold stiffness mismatch or poor cell-seeding |
|
| Poor collagen production in late stage | Inadequate mechanical stimulation or growth factor depletion |
|
| Loss of tenogenic markers after passage | Cell senescence or dedifferentiation |
|
Optimization Tips
As a leading innovator in stem cell technologies, Creative Biolabs provides comprehensive iPSC-derived tendon cell development services tailored to both academic research and pharmaceutical development.
| Service Module | Description |
|---|---|
| Custom Tenocyte Differentiation | iPSC-to-tenocyte differentiation from client-provided or in-house iPSC lines |
| 3D Tendon Construct Engineering | Biofabrication of functional tendon-like tissue using aligned scaffolds |
| Phenotypic & Functional Validation | qPCR, ELISA, immunostaining, biomechanical readouts |
| High-throughput Compound Screening | Screening platform using tendon-like cells for regenerative or anti-fibrotic agents |
The generation of tendon-like cells from iPSCs offers a transformative approach for studying tendon biology and repairing damaged connective tissues. By precisely orchestrating developmental signals and incorporating mechanical cues, high-purity tenocyte-like cells can be produced in vitro. Creative Biolabs is your trusted partner in this journey, offering integrated differentiation, validation, and application platforms tailored to your R&D needs.
For more information or a custom consultation, contact us today.
References
For Research Use Only. Not For Clinical Use.