Induced pluripotent stem cells (iPSCs) offer a powerful platform for generating disease-relevant cell types in vitro. Among these, dopaminergic neurons are particularly valuable for modeling neurodegenerative diseases such as Parkinson's disease (PD), drug screening, and cell replacement therapies. At Creative Biolabs, we leverage well-validated, scalable differentiation protocols to guide iPSCs toward midbrain dopaminergic neuron fate, incorporating precise patterning cues and maturation steps to yield functional and phenotypically relevant neurons.
iPSCs are reprogrammed from somatic cells and possess the capacity to differentiate into virtually any cell type, including region-specific neurons. Using well-defined developmental cues, researchers can recapitulate embryonic midbrain development in vitro, steering iPSCs through neuroectodermal stages toward ventral midbrain dopaminergic fate. This strategy enables the production of dopaminergic neurons with high purity, reproducibility, and scalability.
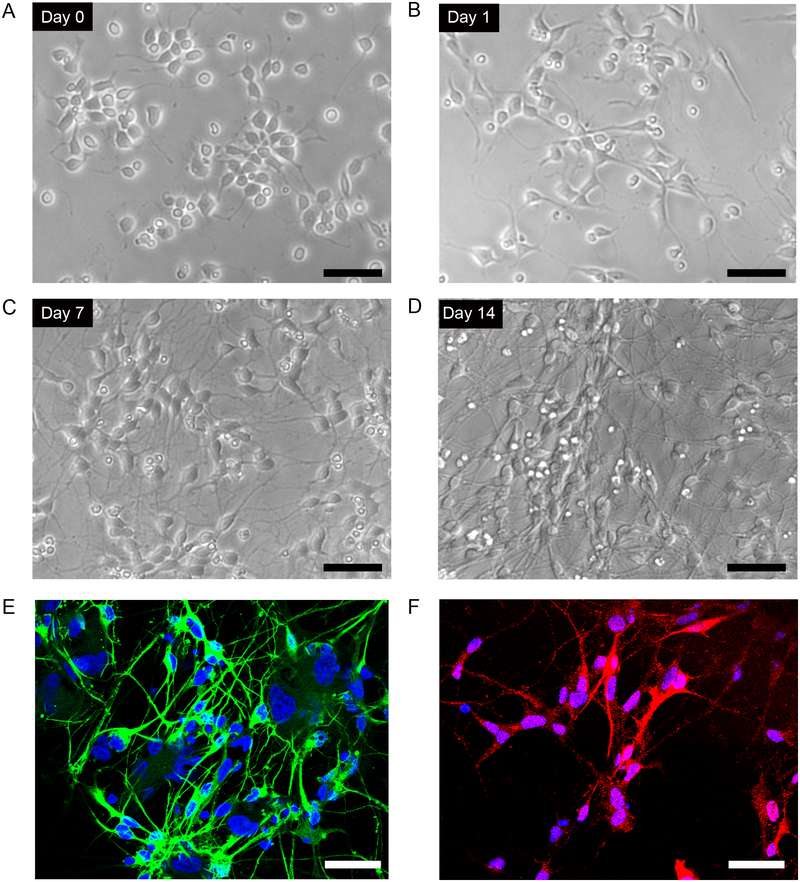 Fig.1 Culture of human iPSC-derived dopaminergic neurons.1,2
Fig.1 Culture of human iPSC-derived dopaminergic neurons.1,2
The in vitro differentiation process typically comprises three key phases:
At Creative Biolabs, we have optimized a robust, scalable protocol for generating ventral midbrain dopaminergic neurons from iPSC lines.
| Reagent | Description |
|---|---|
| Matrigel | A basement membrane matrix that supports stem cell adhesion and maintains their pluripotent state. |
| mTeSR1 medium | A feeder-free medium optimized for the culture and expansion of iPSCs. |
| Neural induction medium | A defined medium for inducing iPSCs into neuroectodermal progenitors during the first week of differentiation. |
| N2 supplement | A supplement for promoting neural progenitor cell survival and growth during early differentiation. |
| B27 supplement | Supports neuronal differentiation and growth, excluding Vitamin A to prevent retinoic acid-induced differentiation. |
| BMP receptor inhibitor | Neural induction by blocking BMP signaling |
| TGF-β receptor inhibitor | Supports neural induction by inhibiting the TGF-β pathway |
| GSK3β inhibitor | Enhances WNT signaling, critical for midbrain patterning and DA neuron differentiation |
| BDNF, GDNF, Ascorbic Acid, cAMP | Key growth factor |
Culture iPSCs on Matrigel in mTeSR1 medium. Use Accutase to gently dissociate colonies and replate at 70–80% confluency. Ensure cells are pluripotent by confirming expression of markers (OCT4, NANOG, SOX2). Avoid overconfluence or spontaneous differentiation before initiating neural induction.

Replace mTeSR1 with neural induction medium. Feed daily with fresh medium. Monitor for morphological changes: cells should transition from iPSC colonies to neural rosette-like structures. Immunostain for PAX6 and SOX1 to confirm early neural fate.

Directing neural progenitors toward a ventral midbrain dopaminergic lineage. Switch to patterning medium. Feed daily, monitor morphology—cells should become denser and more neuroepithelial. Immunostain for OTX2, FOXA2 to verify midbrain identity.

Promote the terminal differentiation and functional maturation of dopaminergic neurons. Replace media every 2 days with maturation medium. Allow cells to mature up to Day 35–50. Typical morphology includes extended neurites and interconnected networks.
At Creative Biolabs, we implement a comprehensive quality control (QC) workflow that integrates phenotypic, molecular, functional, and safety assessments to ensure consistent and reliable neuronal identity.
| Marker | Type | Relevance |
|---|---|---|
| Tyrosine Hydroxylase (TH) | Enzyme | Canonical marker of dopaminergic neurons involved in dopamine biosynthesis. |
| NURR1 | Transcription factor | Critical for dopaminergic neuron identity and midbrain specificity. |
| LMX1A | Transcription factor | Defines ventral midbrain neural progenitors. |
| FOXA2 | Transcription factor | Required for early midbrain floor plate development. |
| βIII-Tubulin (Tuj1) | Structural protein | Pan-neuronal marker indicating neuronal commitment. |
| MAP2 | Cytoskeletal protein | Expressed in mature neurons; used to assess maturation. |
Variability in iPSC quality, reagent lot, cell handling, and environmental factors can all impact the efficiency, purity, and functionality of the final neuronal population. The following guide outlines the most common issues encountered during each stage of differentiation, along with expert-proven troubleshooting solutions and optimization strategies.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low iPSC viability post-passage | Harsh dissociation or inadequate ROCK inhibitor |
|
| Morphological heterogeneity | Mixed iPSC clones or incomplete reprogramming |
|
| Inefficient neural conversion | Suboptimal dual-SMAD inhibition |
|
| Weak expression of midbrain markers | Incorrect CHIR99021 or purmorphamine dosage |
|
| Poor cell expansion | Lack of mitogenic support |
|
| Short or sparse neurites | Incomplete maturation or oxidative stress |
|
| Neurons fail to spike or release dopamine | Immature electrophysiological profile |
|
Creative Biolabs offers technical consultation and protocol customization to help troubleshoot difficult cases. Whether you need to adapt the workflow for specific patient iPSC lines, scale-up for screening campaigns, or overcome low-yield challenges, our team is ready to support your research success.
Creative Biolabs offers a comprehensive suite of stem cell differentiation and neuronal characterization services.
Tailored protocols for neuronal, cardiac, hepatic, and glial lineages.
Generation of integration-free iPSC lines from somatic tissues.
CRISPR/Cas9 knock-in/knock-out strategies for isogenic controls.
For custom protocol design, scalability support, and functional testing services tailored to your pipeline, please contact Creative Biolabs. Together, we accelerate innovation in neurodegenerative disease research and regenerative medicine.
References
Created July 2025
For Research Use Only. Not For Clinical Use.