Skeletal muscle tissue plays a vital role in voluntary movement, posture maintenance, and metabolic regulation. Myopathies, muscular dystrophies, and age-related muscle degeneration highlight the urgent need for in vitro human skeletal muscle models. iPSCs offer a renewable and patient-specific source of cells, which can be differentiated into functional skeletal myocytes using well-defined protocols.
By harnessing key transcriptional regulators such as PAX3, PAX7, and MYOD1, as well as modulating key signaling pathways (WNT, BMP, FGF), Creative Biolabs has developed robust and customizable protocols to generate highly pure and functional skeletal muscle cells from iPSCs.
The differentiation of skeletal muscle cells from human iPSCs represents a complex yet highly orchestrated biological process that emulates embryonic myogenesis. This in vitro strategy provides a scalable, patient-specific, and ethically favorable alternative to primary myoblast isolation
Skeletal myogenesis from iPSCs involves the stepwise recapitulation of mesoderm development, paraxial mesoderm patterning, myogenic lineage specification, and myotube maturation. The success of this process depends on a fine-tuned temporal and spatial activation of key signaling pathways, including:
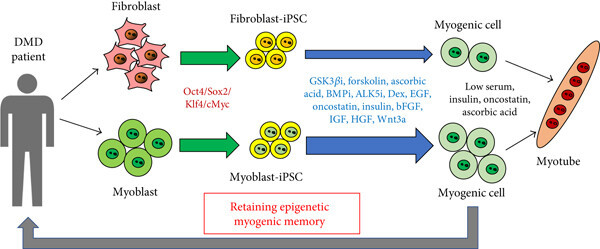 Fig.1 Myogenic cells induced from myoblast-derived iPSCs.1,2
Fig.1 Myogenic cells induced from myoblast-derived iPSCs.1,2
There are two primary strategies for inducing skeletal muscle differentiation from iPSCs.
Uses a cocktail of small molecules and growth factors to sequentially activate or repress developmental pathways. This approach is preferred for clinical translation due to its xeno-free and integration-free nature.
A rapid and efficient method leveraging the forced expression of master myogenic regulators. While highly efficient, it may involve viral vectors or integration events that limit downstream applications.
At Creative Biolabs, we have optimized platforms to ensure high purity, reproducibility, and functional fidelity of derived skeletal muscle cells.
| Component | Details |
|---|---|
| iPSC line | Validated for pluripotency and karyotype stability |
| Basal media | Basement membrane substrate |
| Medium | DMEM/F12, KnockOut Serum Replacement, N2/B27 supplements |
| Growth factors | Activin A, CHIR99021 (WNT agonist), FGF2, IGF-1 |
| Small molecules | SB431542 (TGF-β inhibitor), LDN193189 (BMP inhibitor) |
| Antibodies for characterization | PAX7, MYOD1, Desmin, MHC |
| Matrix components | Matrigel or Laminin-521 |
| Other | ROCK inhibitor, TrypLE Express, CellTracker dyes |
Culture iPSCs on Matrigel-coated plates in mTeSR1 medium. When colonies reach ~70% confluency, pre-treat with Y27632 for 1 hour before single-cell dissociation. Plate cells and allow 24 hours for recovery.

Replace medium with mesoderm induction medium: DMEM/F12 + CHIR99021 + FGF2. Culture for 4 days, change medium daily. Monitor expression of mesodermal markers: Brachyury, Tbx6.

Switch to specification medium: DMEM/F12 + horse serum + SB431542 + LDN193189. Add IGF-1 to promote myogenic lineage commitment. Check for expression of PAX7, MYF5, and Desmin.

Culture in high-glucose DMEM + FBS + FGF2. Allow proliferation of myoblasts, maintaining sub-confluency. Passage cells 1–2 times if needed.

Culture in differentiation medium (DMEM + horse serum, no growth factors). Myotube formation begins within 3–5 days. Confirm via Myosin Heavy Chain (MHC) and Titin expression.
We implement stringent quality control checks throughout the differentiation process, including:
Skeletal muscle cell differentiation from iPSCs involves multiple tightly regulated steps, each vulnerable to variations in culture conditions, reagent activity, and cell behavior. Below is a comprehensive troubleshooting guide and expert-level optimization strategies to maximize success.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low efficiency of mesoderm induction | Inactive CHIR99021 or poor cell viability |
|
| Heterogeneous myogenic commitment | Inconsistent WNT/BMP modulation or batch variability in supplements |
|
| Myoblast proliferation failure | Lack of FGF2, suboptimal glucose or serum levels |
|
| Poor myotube fusion | Over-confluent or under-confluent seeding, matrix inconsistency |
|
| Apoptosis during stage transitions | Abrupt media switch or stress-induced cell detachment |
|
| Myotubes lack contractility | Incomplete maturation or insufficient stimulus |
|
Creative Biolabs offers an extensive portfolio of iPSC-based research solutions and customized stem cell differentiation services.
Generation of skeletal muscle cells from iPSCs.
Gene knockout, knock-in, or point mutation of iPSC lines.
Generation of patient-specific or disease-specific iPSC lines from PBMCs, fibroblasts, or urine-derived cells. Reprogramming via virus, episomal vectors, or mRNA.
The generation of skeletal muscle cells from iPSCs is a promising platform that enables researchers to model complex muscle diseases and develop novel therapeutic approaches. Creative Biolabs is proud to support your journey with high-fidelity protocols, validated reagents, and comprehensive services that ensure success from iPSC to fully functional myotubes.
For tailored support or to initiate your skeletal myogenesis project, contact our experts today.
References
For Research Use Only. Not For Clinical Use.