Endothelial progenitor cells (EPCs) play a pivotal role in vasculogenesis and vascular repair. These cells have demonstrated potential in regenerative medicine for treating ischemic cardiovascular diseases, wound healing, and vascular tissue engineering. With the advent of induced pluripotent stem cell (iPSC) technologies, deriving EPCs from iPSCs offers a scalable and patient-specific alternative to traditional sources.
At Creative Biolabs, we provide a highly standardized, efficient, and reproducible protocol for generating EPCs from human iPSCs. Our service ensures phenotypic fidelity, functional competence, and compatibility with downstream applications.
EPCs are a population of cells capable of differentiating into mature endothelial cells and contributing to neovascularization in response to ischemic injury or tissue regeneration demands. However, access to consistent, well-characterized EPC populations from peripheral blood or bone marrow remains challenging due to donor variability, low abundance, and rapid loss of regenerative potential upon in vitro expansion.
iPSCs provide a robust, renewable, and patient-specific source of progenitor cells that can be directed toward endothelial lineages under defined culture conditions. Generating functionally competent EPCs from iPSCs requires precise orchestration of developmental signaling pathways, including mesodermal patterning, vascular commitment, and endothelial maturation.
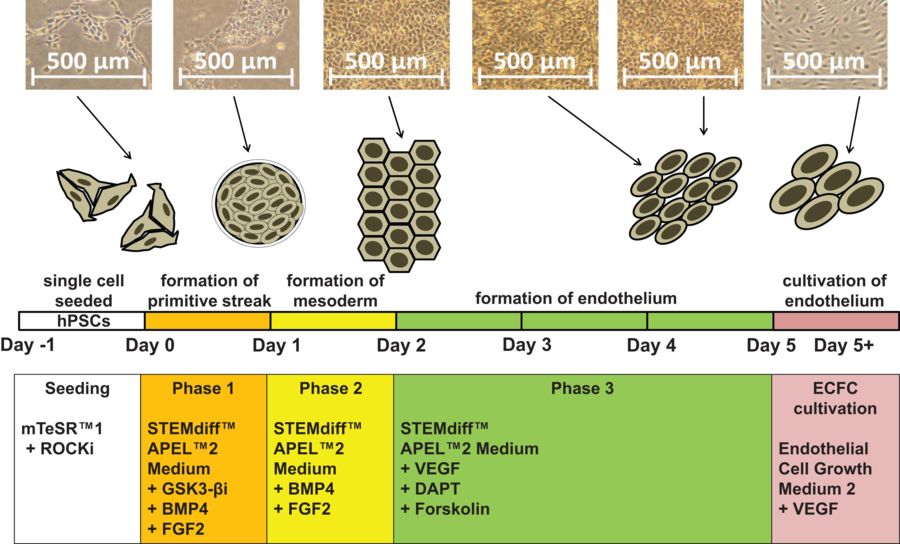 Fig.1 hPSCs differentiated into endothelium in three phases.1,2
Fig.1 hPSCs differentiated into endothelium in three phases.1,2
Our service leverages high-purity iPSC lines, matrix-guided differentiation, and fine-tuned cytokine cocktails to ensure high yield, phenotypic stability, and functional competence of derived EPCs.
| Component | Details |
|---|---|
| iPSCs | Validated, feeder-free |
| Matrigel or vitronectin coating | Basement membrane substrate |
| Medium |
Equivalent stem cell maintenance medium Endothelial cell growth medium-2 (EGM-2) |
| Induction factors |
BMP-4 Activin A VEGF-A bFGF |
| Others |
Accutase ROCK inhibitor PBS Penicillin-Streptomycin Trypan Blue |
Subculture iPSCs on Matrigel-coated plates in medium. Maintain iPSCs at ~80% confluence. Passage cells every 3–4 days using Accutase. Add ROCK inhibitor for 24 hours post-splitting to enhance survival. Ensure iPSCs exhibit a compact colony morphology with high nucleus-to-cytoplasm ratio and express pluripotency markers (OCT4, SOX2, NANOG).

Replace the stem cell medium with mesoderm induction medium. Incubate for 48 hours. Monitor morphological changes: cells should appear flattened and form a monolayer. Assess mesoderm markers (e.g., Brachyury/T) by qPCR or immunostaining.

Replace medium with EGM-2 supplemented with VEGF-A. Continue daily medium changes for 4–5 days. Cells will gradually exhibit cobblestone morphology, a hallmark of endothelial identity. By day 7, cells begin expressing EPC markers such as CD34 and KDR (VEGFR2).

Harvest cells using Accutase. Optional purification by MACS or FACS using CD34 or CD31 microbeads/antibodies. Seed purified EPCs in EGM-2 medium on fibronectin-coated plates. Expand EPCs for up to 3 passages for downstream applications.
To ensure consistency, functionality, and applicability of iPSC-derived EPCs across diverse research contexts, Creative Biolabs implements a multi-tiered quality control framework. Our QC workflow integrates molecular, cellular, and functional assessments.
| Evaluation Category | Assay Type | Expected Outcome |
|---|---|---|
| Phenotypic Validation | Flow Cytometry Markers |
|
| Molecular Characterization | qPCR Panel |
|
| Functional Testing |
Tube Formation Assay Acetylated LDL Uptake Assay Nitric Oxide (NO) Production Assay |
|
| Problem | Possible Cause | Solution |
|---|---|---|
| Low mesoderm induction efficiency | Degraded BMP-4 or Activin A; poor cell density |
|
| Low EPC yield | Suboptimal VEGF concentration or exposure time |
|
| Cell death during transition | Harsh detachment; absence of ROCK inhibitor |
|
| Heterogeneous cell population | Inadequate purification step |
|
| Weak tube formation | Incomplete differentiation; stress during expansion |
|
| Persistent pluripotency marker expression | Incomplete lineage commitment |
|
We have established several optimization strategies to enhance EPC quality and scalability.
Creative Biolabs offers an extensive portfolio of iPSC-based vascular research solutions and customized cell differentiation services.
Generation of mature endothelial cells (ECs) from human iPSCs with validated markers (CD31, CD144, vWF) and functional tube formation capacity. Ideal for vascular permeability assays and inflammation models.
Directed differentiation into contractile or synthetic phenotype VSMCs from iPSCs. Applications include vascular disease modeling, tissue engineering, and drug screening.
Gene knockout, knock-in, or point mutation of iPSC lines to study gene function in vascular development or simulate disease phenotypes. Integration with EPC differentiation available.
Generation of patient-specific or disease-specific iPSC lines from PBMCs, fibroblasts, or urine-derived cells. Reprogramming via virus, episomal vectors, or mRNA.
Creative Biolabs offers flexible, tailored solutions for researchers pursuing iPSC-derived endothelial lineages. We are ready to support your innovation. Contact us today to discuss your project or request a custom quote.
References
For Research Use Only. Not For Clinical Use.