Cardiomyocytes derived from induced pluripotent stem cells (iPSCs) mimic the functional, molecular, and electrophysiological properties of native cardiac cells, providing a powerful platform for drug screening, disease modeling, and regenerative therapy development. At Creative Biolabs, we offer a comprehensive and scientifically validated protocol for the efficient and reproducible generation of cardiomyocytes from iPSCs, tailored to meet the demands of translational and preclinical research.
The differentiation of iPSCs into cardiomyocytes is typically achieved through the sequential modulation of key signaling pathways, which mirror embryonic heart development.
Initially, iPSCs are guided to exit the pluripotent state and commit to the mesoderm lineage, which serves as the germ layer precursor to the cardiovascular system. This mesoderm induction stage is critically dependent on transient activation of the Wnt signaling pathway, most commonly achieved using small molecules. Once the mesoderm identity is established, the cells are then exposed to Wnt inhibitors to suppress posterior patterning and steer differentiation toward anterior mesoderm and cardiac mesoderm.
As differentiation progresses, the cells enter the cardiac progenitor stage, expressing key transcription factors such as MESP1, NKX2.5, and ISL1, which prime the genome for downstream cardiac gene expression. Subsequently, in the cardiac specification phase, structural and functional cardiomyocyte proteins are upregulated, and the cells begin to exhibit hallmark contractile activity.
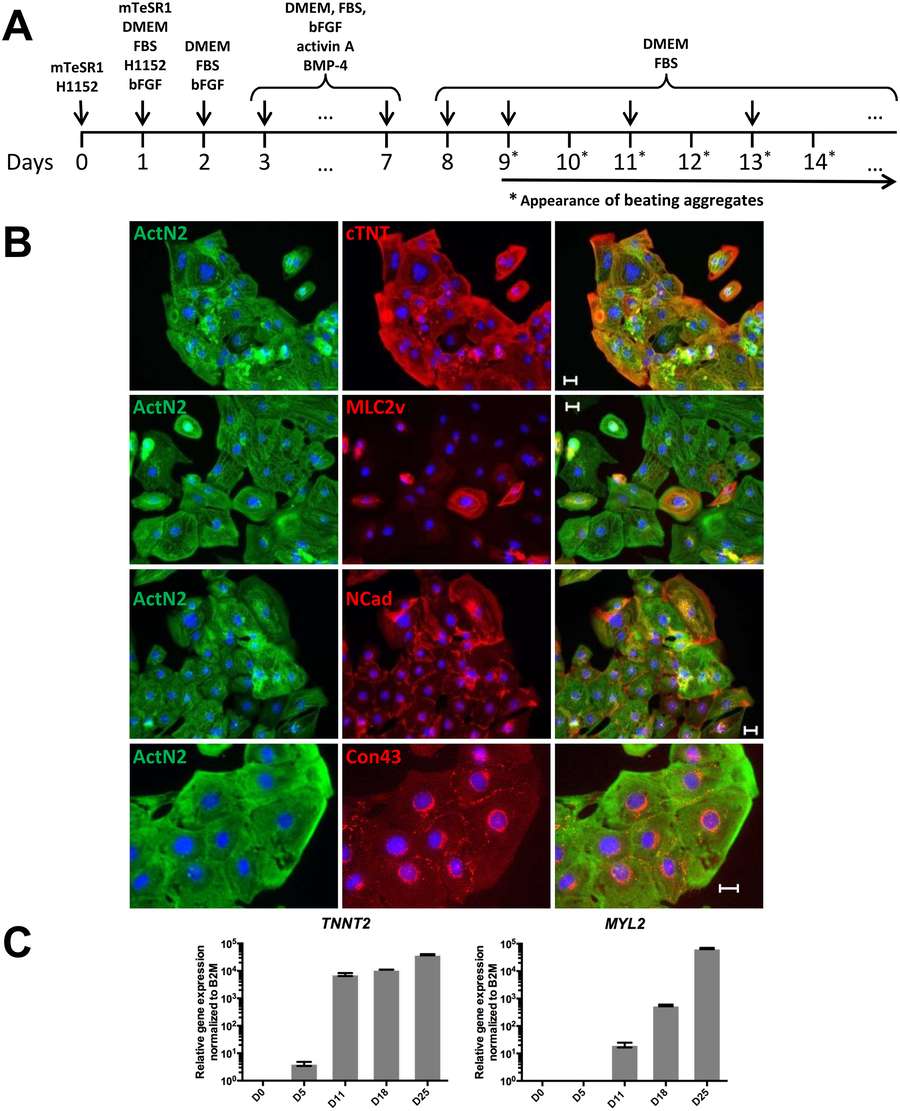 Fig.1 Differentiation and characterization of iPS cell-derived cardiomyocytes.1,2
Fig.1 Differentiation and characterization of iPS cell-derived cardiomyocytes.1,2
Through precise modulation of signaling pathways and proprietary medium formulations, we routinely achieve >85% cardiomyocyte purity across a variety of iPSC lines.
| Component | Specification |
|---|---|
| iPSC line | Well-characterized, mycoplasma-free |
| Matrigel or vitronectin | For coating culture surfaces |
| Medium | For iPSC maintenance |
| RPMI 1640 medium | For differentiation |
| B27 supplement | For supporting differentiation stages |
| CHIR99021 | GSK3β inhibitor to activate Wnt signaling |
| IWP-2 or IWR-1 | Wnt signaling inhibitor |
| ROCK inhibitor | For enhancing cell survival during passaging |
| PBS, TrypLE, FBS, and Pen-Strep | Standard cell culture reagents |
Culture human iPSCs on Matrigel-coated plates in medium. Maintain cells in a pluripotent state. Passage cells using EDTA or TrypLE when reaching 80-90% confluence. Precondition cells with ROCK inhibitor prior to plating for differentiation.

Replace maintenance medium with RPMI + B27 minus insulin. Add CHIR99021 for 24 hours to activate canonical Wnt signaling and incubate.

Remove CHIR99021-containing medium. Add RPMI + B27 minus insulin + IWP-2 or IWR-1. This stage represses Wnt signaling to guide cells toward cardiac lineage.

Replace medium with RPMI + B27 (with insulin) every 2–3 days. Beating clusters of cardiomyocytes typically appear between Day 7 and 10. Enrich cardiomyocytes using lactate-based metabolic selection.
All cardiomyocyte preparations undergo rigorous quality control to ensure identity, purity, and function.
Achieving high-efficiency cardiomyocyte differentiation from iPSCs requires precise control of culture conditions, timing, and reagent quality.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low mesoderm induction efficiency | Suboptimal CHIR99021 concentration or expired reagents |
|
| Poor cell survival after replating | No ROCK inhibitor or rough mechanical dissociation |
|
| Inconsistent beating across wells | Uneven matrix coating or cell seeding density |
|
| No visible contraction after Day 10 | Incorrect Wnt inhibition window or incomplete Wnt suppression |
|
| Mixed or non-cardiac populations | Incomplete mesoderm commitment or loss of lineage-specific cues |
|
| Spontaneous detachment of cells | Over-confluent culture or weak matrix adhesion |
|
| Low cTnT+ purity in flow cytometry | Incomplete differentiation or poor B27 supplement quality |
|
Optimization Tips
Creative Biolabs offers end-to-end support in cardiomyocyte-related projects, including:
Tailored protocols for specific cardiomyocyte subtypes (atrial, ventricular, pacemaker cells).
Gene knockout, knock-in, or point mutation of iPSC lines to generate disease models for cardiac research.
High-throughput screening of compounds using MEA and calcium flux assays.
The generation of cardiomyocytes from iPSCs offers unprecedented opportunities to model human cardiac physiology in a dish. With Creative Biolabs' robust and customizable differentiation platform, researchers gain access to highly pure, functionally competent cardiomyocytes ideal for disease modeling and drug discovery.
References
For Research Use Only. Not For Clinical Use.