Renal progenitor cells (RPCs) are multipotent precursors essential for kidney development and regeneration. These cells hold significant potential in nephrotoxicity modeling, drug screening, and regenerative medicine. Induced pluripotent stem cells (iPSCs) provide an unlimited source for generating RPCs, offering a controllable and reproducible system for studying nephrogenesis and modeling kidney diseases.
Creative Biolabs leverages years of stem cell differentiation expertise to offer optimized, customizable protocols for generating renal progenitor cells from iPSCs, supporting applications in drug testing, nephrology research, and renal tissue engineering.
The generation of RPCs from iPSCs represents a pivotal advancement in kidney biology, toxicology, and regenerative medicine. This strategy recapitulates key developmental stages of nephrogenesis through the sequential activation of signaling pathways that guide iPSCs toward intermediate mesoderm and eventually into nephron progenitor cell fate.
In the human embryo, kidney development initiates from the intermediate mesoderm, which gives rise to the metanephric mesenchyme and ureteric bud—the two essential components of the mature nephron structure. During in vitro differentiation, this developmental logic is mimicked by carefully timed exposure to growth factors, which collectively regulate lineage specification, patterning, and progenitor stabilization.
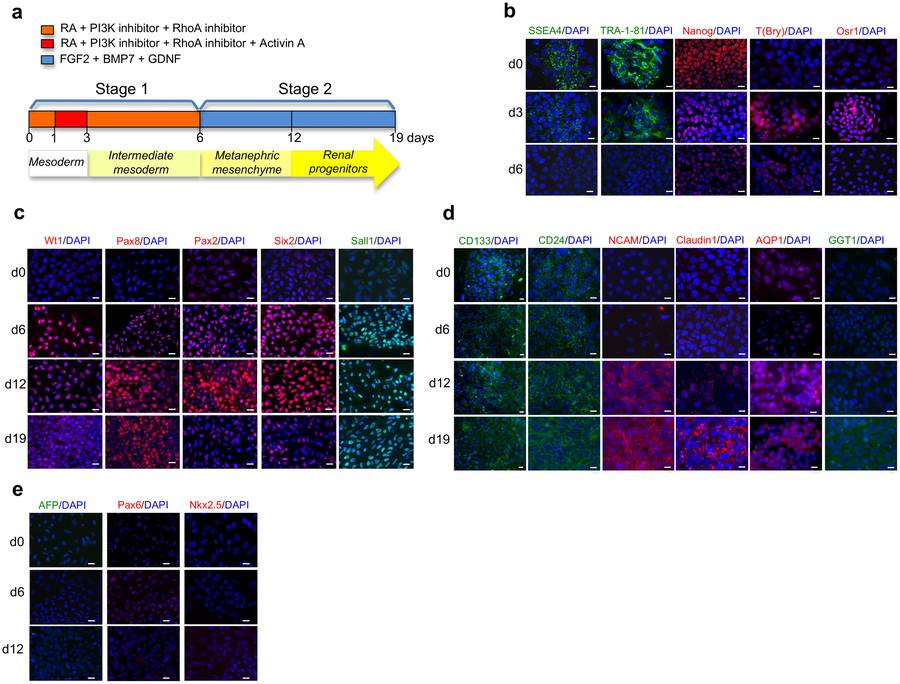 Fig.1 Stepwise differentiation of human iPSCs towards RPCs.1,2
Fig.1 Stepwise differentiation of human iPSCs towards RPCs.1,2
By optimizing cytokine concentrations, timing, and extracellular matrix support, this protocol allows for reproducible generation of RPCs with high purity and functionality. These cells can then be expanded, cryopreserved, or used directly in downstream applications.
| Component | Details |
|---|---|
| iPSC Line | Feeder-free, karyotypically normal |
| Matrigel or Vitronectin | For iPSC culture |
| Medium | For iPSC maintenance |
| Differentiation medium | RPMI 1640 + B27 supplement (minus insulin) |
| Others | Activin A, BMP4, CHIR99021, FGF9, Heparin, PBS |
iPSCs are maintained under feeder-free conditions on Matrigel-coated plates using medium. Split cells at 70–80% confluency using cell dissociation agents. Ensure expression of pluripotency markers (OCT4, NANOG, SOX2).

Replace iPSC medium with RPMI + B27 minus insulin, supplemented with CHIR99021 and BMP4. Incubate for 48 hours to induce primitive streak and mesodermal lineage. Confirm Brachyury (T) and MIXL1 expression by qPCR or immunofluorescence.

Replace medium with RPMI + B27 (minus insulin), supplemented with Activin A and CHIR99021 (reduced concentration). Incubate for 2–3 days to guide differentiation into intermediate mesoderm. Confirm expression of PAX2, OSR1, and LHX1.

Supplement medium with FGF9 and Heparin to drive nephron progenitor fate. Maintain cells for 4–5 days with daily medium change. Evaluate expression of WT1, SIX2, CITED1, and HOXD11 by flow cytometry or immunostaining.

Detach RPCs, reseed in Matrigel-coated plates with FGF9 to expand cell numbers. Maintain for up to 10 additional days for downstream applications.
To ensure protocol fidelity and clinical relevance, Creative Biolabs applies comprehensive quality assessments.
| Parameter | Methods |
|---|---|
| Morphology | Phase contrast microscopy |
| Marker Expression | Immunostaining, qPCR, Flow cytometry |
| Purity | % of WT1+/SIX2+ cells |
| Pluripotency Loss | OCT4/NANOG downregulation |
| Karyotyping | G-banding or SNP array |
| Mycoplasma Testing | PCR-based assay |
This section provides an expanded troubleshooting guide along with practical optimization strategies to address commonly encountered issues.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low mesoderm induction efficiency | Ineffective Wnt activation due to CHIR99021 degradation |
|
| Cell detachment or death during early induction | Over-confluent iPSC seeding or harsh media shift |
|
| Heterogeneous populations at IM or RPC stages | Non-uniform cytokine distribution or inconsistent cell density |
|
| Reduced expression of SIX2/WT1 | Incomplete IM specification or FGF9 inactivation |
|
| Premature spontaneous differentiation | Overgrowth, prolonged culture without passaging |
|
| Low cell viability post-passaging | Over-trypsinization or poor ECM coating |
|
| Batch variability | Lot-to-lot differences in B27 or cytokines |
|
Optimization Tips for Enhanced Performance
At Creative Biolabs, we provide an end-to-end solution suite for renal and stem cell research.
We offer efficient, non-integrative iPSC generation from various somatic sources (e.g., PBMCs, fibroblasts), followed by thorough characterization to ensure pluripotency and genomic integrity.
Beyond RPCs, we support downstream differentiation into more mature renal cell types and 3D kidney structures.
We provide gene editing services to introduce or correct kidney disease-relevant mutations in iPSCs or RPCs, enabling isogenic controls and disease modeling.
Generating renal progenitor cells from iPSCs presents a robust, scalable, and ethically sustainable model for kidney-related research and therapeutic development. Creative Biolabs stands at the forefront of iPSC differentiation services, combining scientific rigor with customized flexibility to meet your experimental needs. Contact us today to accelerate your renal research innovations.
References
For Research Use Only. Not For Clinical Use.