The generation of erythroid cells from human induced pluripotent stem cells (iPSCs) provides a robust in vitro platform for studying erythropoiesis, hemoglobinopathies, and drug screening. Compared to primary cells, iPSC-derived erythroid cells offer unlimited supply, genetic consistency, and scalability. Creative Biolabs offers a highly optimized, feeder-free, and serum-free protocol that ensures the efficient differentiation of iPSCs into erythroid progenitors and terminal erythrocytes.
During natural hematopoiesis, erythroid cells arise from hematopoietic stem and progenitor cells (HSPCs) in the bone marrow. Mimicking this process from iPSCs requires a staged differentiation protocol that sequentially induces mesoderm, hemogenic endothelium, hematopoietic progenitors, and finally, erythroid cells. Proper modulation of signaling pathways such as BMP, Wnt, Notch, and VEGF is essential to recapitulate embryonic and definitive hematopoietic waves.
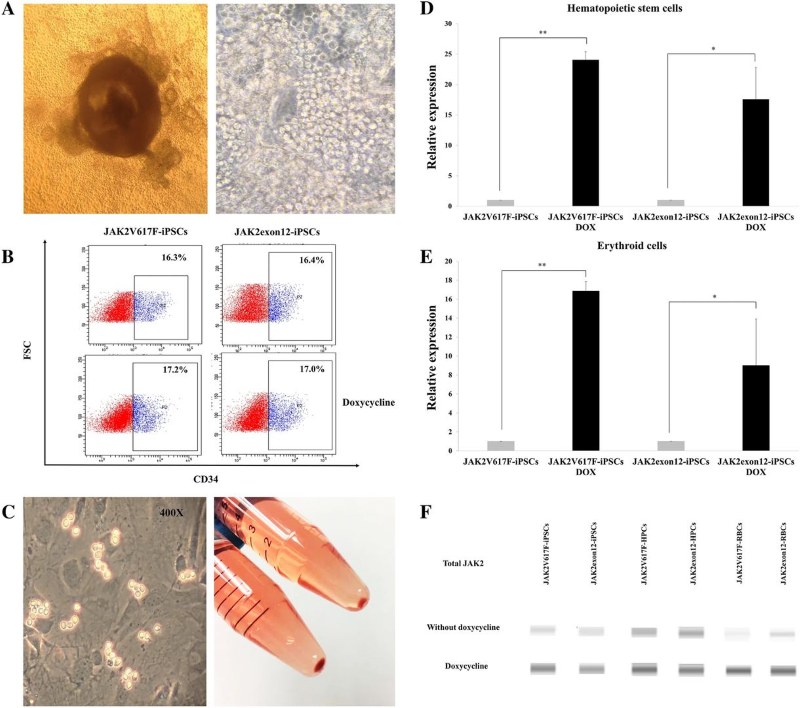 Fig.1 Erythroid cell differentiation from modified iPSCs.1,2
Fig.1 Erythroid cell differentiation from modified iPSCs.1,2
iPSC-derived erythroid cells have found increasing relevance in both fundamental and translational research.
At Creative Biolabs, we have leveraged years of stem cell differentiation expertise to develop a robust, scalable, and customizable erythroid differentiation platform.
| Category | Reagents/Materials | Description |
|---|---|---|
| Cell Culture | iPSC lines | Human-derived, fully characterized |
| Medium | iPSC expansion medium | |
| IMDM, RPMI-1640 | For hematopoietic differentiation | |
| Cytokines & Small Molecules | BMP4, VEGF, SCF, IL-3, EPO, TPO, FLT3L, IL-6 | >95% purity |
| Dexamethasone, Heparin | Differentiation enhancers | |
| Supplements | Ascorbic acid, Penicillin/Streptomycin | Standard formulations |
| Others | Flow cytometry antibodies (CD34, CD43, CD71, CD235a) | Fluorophore-conjugated |
| Hematopoietic colony-forming assay kit | CFU-E/BFU-E detection |
Plate iPSCs on Matrigel-coated plates. Maintain cells in medium with daily media changes. Monitor morphology; passage before confluence exceeds 80%.

Replace with RPMI-1640 + B27 (without insulin) + Activin A + BMP4. Incubate at 37°C, changing medium daily.

Switch to RPMI + B27 + VEGF, SCF, FLT3-L, and IL-6. Promote CD34⁺CD43⁺ hematopoietic progenitor development.

Harvest hematopoietic progenitors via gentle dissociation. Culture in IMDM + 15% BIT9500 + SCF, EPO, and IL-3.

Continue erythroid maturation with EPO, transferrin, and insulin. Monitor CD71 (transferrin receptor) and Glycophorin A (GlyA) expression via flow cytometry.
Rigorous quality control (QC) is essential to ensure the identity, purity, functionality, and reproducibility of iPSC-derived erythroid cells. Creative Biolabs employs multi-parametric assessments across phenotypic, molecular, and functional levels to ensure high-quality cell outputs.
| Analysis | Description |
|---|---|
| Morphological Evaluation |
|
| Flow Cytometry |
We monitor surface marker expression during each differentiation stage:
|
| Hemoglobin Composition Analysis |
|
In erythroid differentiation from iPSCs, variability can arise from donor iPSC line heterogeneity, reagent quality, and culture microenvironment. Here are common issues and solutions.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low mesoderm induction efficiency | iPSCs are too confluent or stressed |
|
| Poor hematopoietic specification (low CD34⁺) | Suboptimal cytokine gradients or expired factors |
|
| High cell death during transition to erythroid stage | Osmotic shock, abrupt medium change |
|
| Low GlyA⁺ cell population | Incomplete maturation, inadequate EPO/SCF |
|
| Low enucleation efficiency | Suboptimal density or lack of supportive factors |
|
| Batch-to-batch variation | Inconsistent matrix or supplement quality |
|
Improving erythroid yield and function from iPSCs involves fine-tuning of culture conditions, timing, and supplementation strategies. Here are the best practices derived from our extensive project experience.
As a global leader in stem cell research and differentiation platforms, Creative Biolabs offers a comprehensive suite of services to support erythroid cell generation from iPSCs and beyond. Our integrated solutions enable customized, scalable, and GMP-compliant project execution for both academic and industrial clients.
To explore how Creative Biolabs can accelerate your hematopoietic research or biomanufacturing pipeline, visit our stem cell solutions or get in touch with our experts for a customized project proposal.
References
For Research Use Only. Not For Clinical Use.