Induced pluripotent stem cells (iPSCs) offer a patient-specific, renewable source for differentiation into all three germ layers. Among the most ambitious and biologically significant objectives is the derivation of functional germ cells—spermatozoa and oocytes—from iPSCs. Such technology creates a platform for studying germline development, epigenetic reprogramming, and transgenerational inheritance in vitro.
Creative Biolabs, leveraging over two decades of stem cell research expertise, offers a cutting-edge, validated protocol for directing the differentiation of iPSCs into germ cells under defined and reproducible conditions.
The in vitro generation of germ cells from iPSCs aims to recapitulate the complex developmental trajectory of primordial germ cells and their maturation into functional gametes entirely outside the body and under controlled laboratory conditions. As a multi-step, temporally coordinated differentiation process, it requires precise modulation of morphogenetic signals, mimicking embryonic germline specification.
A variety of molecular pathways orchestrate this process:
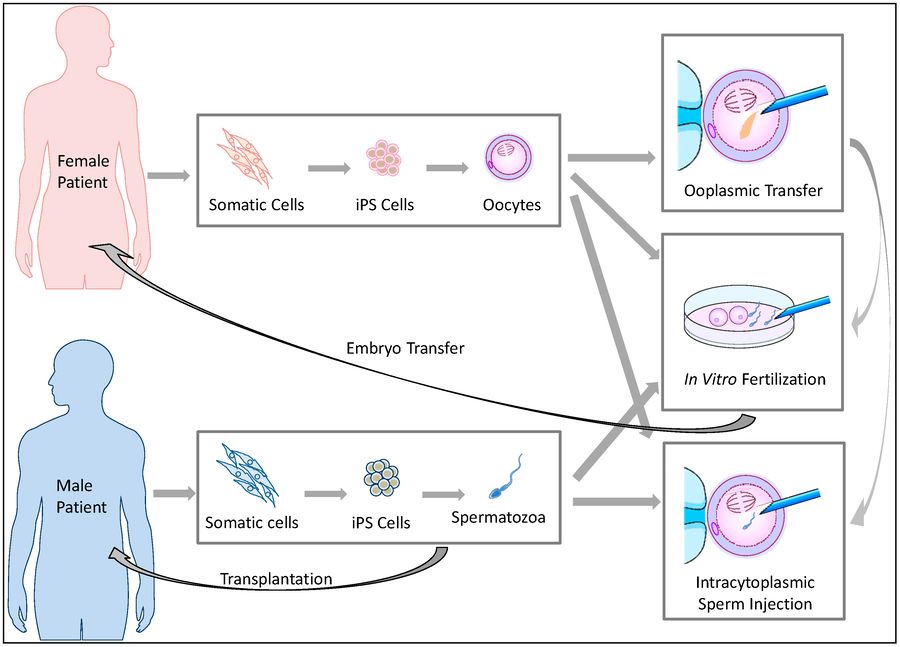 Fig.1 The potential reproductive uses of iPS cell-based germ cells.1,2
Fig.1 The potential reproductive uses of iPS cell-based germ cells.1,2
Creative Biolabs' protocol has been optimized to precisely modulate these pathways using recombinant factors and small molecules in defined, xeno-free conditions.
| Component | Specification |
|---|---|
| iPSC culture medium | mTeSR™1 or equivalent |
| Matrigel or Geltrex | Basement membrane matrix for coating |
| bFGF and Activin A | For EpiLC induction |
| BMP4, LIF, SCF, and EGF | For PGCLC induction |
| Retinoic acid (RA) | Germ cell maturation enhancer |
| FBS and serum replacement | For serum-based induction conditions |
| Anti-TRA-1-60, OCT4, SOX17 antibodies | For pluripotency and germ cell characterization |
| Flow cytometry reagents | For PGCLC sorting |
Plate iPSCs on Matrigel-coated plates. Cultivate for 24 hours in mTeSR™1 medium. Replace medium with EpiLC induction medium (N2B27 + bFGF + Activin A). Incubate for 48 hours, changing media daily. Monitor morphology; EpiLCs appear flatter and more dispersed.

Detach EpiLCs and aggregate in low-attachment U-bottom 96-well plates (embryoid body formation). Use PGCLC induction medium supplemented with BMP4, LIF, SCF, and EGF. Incubate aggregates for 4–6 days. Analyze PGCLC markers using immunocytochemistry or flow cytometry.

Transfer PGCLCs to adherent culture in differentiation medium + RA for 7–14 days. Monitor for expression of DDX4 (VASA), DAZL, SCP3, or STRA8 to assess meiosis initiation. Validate by qPCR, ICC, or RNA-seq.
Creative Biolabs implements rigorous quality assessment at each step of germ cell differentiation.
The process of differentiating iPSCs into germ cells is intricate, with each stage sensitive to variations in culture conditions, reagent quality, and timing. Ensuring consistent results requires proactive troubleshooting and optimization. Below is a detailed guide to address common technical issues and enhance differentiation efficiency.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low EpiLC induction efficiency |
|
|
| Poor PGCLC differentiation |
|
|
| High cell death in aggregates |
|
|
| Lack of PGCLC marker expression |
|
|
| Incomplete meiosis or poor VASA/DDX4 expression |
|
|
| High inter-batch variabilit |
|
|
We are dedicated to accelerating germ cell research through a suite of specialized services tailored to the full workflow of iPSC-to-gamete differentiation. Our platform integrates advanced gene editing, lineage-specific differentiation, multi-omic analysis, and functional validation, empowering clients in academia and industry.
Precise genome modifications for germline development genes;
Introduction of germ cell-specific fluorescent reporters;
Generation of patient-specific iPSC lines for infertility or germline disease research
Fully customizable differentiation protocols based on species, cell line, and target stage;
Development of optimized support systems using testicular or ovarian somatic cells;
Generation of patient-specific or disease-specific iPSC lines from PBMCs, fibroblasts, or urine-derived cells. Reprogramming via virus, episomal vectors, or mRNA.
By implementing a stage-specific, growth-factor-driven approach, Creative Biolabs enables robust, reproducible generation of functional germline cells tailored to your research goals. With our interdisciplinary expertise and state-of-the-art platforms, we offer not only technical execution but also strategic consultation to help you navigate complex questions in reproductive biology.
References
For Research Use Only. Not For Clinical Use.