Natural killer (NK) cells are cytotoxic lymphocytes of the innate immune system. Recent advances in regenerative medicine have enabled the derivation of functional NK cells from induced pluripotent stem cells (iPSCs). This protocol outlines a stepwise procedure for the efficient differentiation of human iPSCs into functional NK cells, covering feeder-based expansion, cytokine modulation, and phenotypic characterization.
NK cells are key effector lymphocytes of the innate immune system, responsible for the rapid identification and elimination of virus-infected, transformed, and stressed cells. Unlike cytotoxic T lymphocytes, NK cells function without prior antigen sensitization and play crucial roles in immune surveillance, tumor immunology, and immunoregulatory balance. The generation of NK cells from iPSCs offers a transformative platform for both basic and applied research due to the inherent advantages of iPSCs—pluripotency, scalability, and the potential for autologous or universal donor-derived immune cell products.
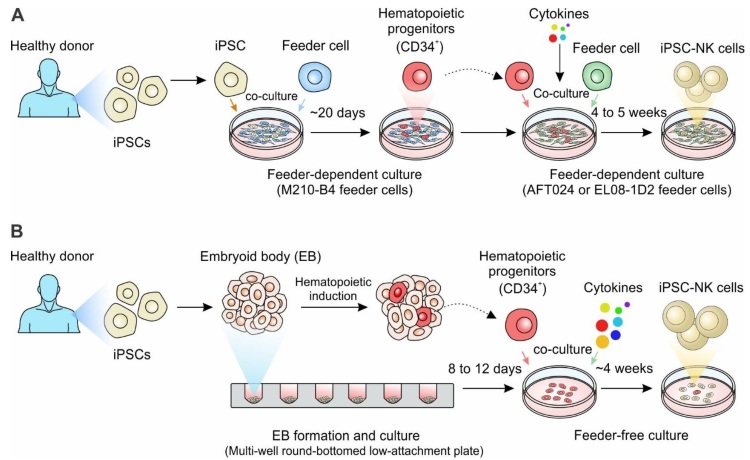 Fig.1 Generation of iPSC-derived NK cell-based cell therapy.1,2
Fig.1 Generation of iPSC-derived NK cell-based cell therapy.1,2
In recent years, iPSC-derived NK (iPSC-NK) cells have gained significant attention as next-generation immune effectors for cell-based assays, disease modeling, and preclinical validation of immune-modulating therapies. Compared to peripheral blood- or cord blood-derived NK cells:
At Creative Biolabs, we have established a robust and modular workflow for deriving functional NK cells from human iPSCs, supporting a variety of downstream applications.
| Category | Reagents/Materials | Description |
|---|---|---|
| iPSC Culture | Matrigel, mTeSR1 Medium | For iPSC maintenance |
| Embryoid Body Formation | RPMI-1640, B27, BMP4, VEGF, SCF | Mesoderm induction |
| Hematopoietic Differentiation | IL-3, IL-6, TPO, Flt3L | To yield CD34⁺ hematopoietic progenitors |
| NK Lineage Induction | IL-15, IL-7, SCF, Flt3L, IL-21 | To drive NK cell commitment |
| Feeder Cells | AFT024 stromal cells or OP9-DL1 | Optional co-culture for NK maturation |
| Analysis | Flow cytometry antibodies (CD45, CD56, CD16, NKG2D), ELISA kits | Phenotypic and functional assays |
Maintain pluripotent stem cells under feeder-free, xeno-free conditions. First, plate iPSCs on Matrigel-coated dishes in mTeSR1 medium. Passage every 3–5 days. Confirm pluripotency markers (OCT4, NANOG, SSEA4) via flow cytometry or immunofluorescence.

Use plates to generate uniform EBs. Culture EBs in RPMI-1640 + B27 supplement with BMP4, VEGF, and SCF. Induce mesodermal lineage markers (e.g., Brachyury, KDR).

Transfer EBs to low-attachment dishes in hematopoietic induction medium containing IL-3, IL-6, TPO, SCF and Flt3L. Monitor the emergence of CD34⁺CD45⁺ progenitor populations using flow cytometry.

Culture hematopoietic progenitors with a defined cytokine cocktail: IL-15, IL-7, IL-21, SCF, and Flt3L. Optionally, co-culture with irradiated AFT024 or OP9-DL1 feeder cells to enhance NK development.

Expand committed NK precursors in medium supplemented with IL-2 and IL-15. Assess for surface markers CD45⁺CD56⁺CD3⁻ and expression of NK functional receptors (e.g., NKG2D, NKp30, NKp44, NKp46).
Robust quality control (QC) and characterization of iPSC-derived NK cells are essential for ensuring reproducibility, functionality, and suitability for downstream applications such as drug screening or immunotoxicity assays. At Creative Biolabs, we implement multi-parameter quality assessments encompassing phenotypic, functional, and genetic validation
| Analysis | Description |
|---|---|
| Phenotypic Analysis |
Flow cytometry: Use multicolor panels to confirm NK-specific surface markers:
Maturation status: CD16 expression reflects cytotoxic maturation; CD62L and KIRs indicate functional heterogeneity. |
| Functional Assays |
|
| Genomic Stability |
|
Effective troubleshooting is crucial to avoid batch failures and ensure consistency. Below is a practical guide to resolving common technical challenges encountered during iPSC-NK differentiation.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low iPSC viability post-passaging | Enzymatic dissociation too harsh or prolonged |
|
| Poor EB formation | Cell clumping or uneven aggregation |
|
| Insufficient CD34⁺ output | Suboptimal mesoderm induction |
|
| Low NK differentiation rate | Cytokine degradation or feeder cell overgrowth |
|
| Loss of CD56 expression during expansion | Excessive IL-2 or culture stress |
|
| Variable cytotoxicity results | Cell aggregation or inconsistent target cell ratio |
|
To maximize efficiency and consistency in iPSC-derived NK cell production, the following strategies are recommended.
We offer fully integrated services to support your iPSC-to-NK workflow.
Generation of GMP-grade iPSCs from PBMCs or fibroblasts
Custom protocols for hematopoietic and NK cell generation
Comprehensive flow cytometry and cytokine profiling
For further customization or large-scale NK cell production, contact our technical specialists to design a tailored solution.
References
For Research Use Only. Not For Clinical Use.