Melanocytes are pigment-producing cells primarily located in the skin, hair follicles, eyes, and inner ear, responsible for synthesizing melanin, which plays a protective role against ultraviolet (UV) radiation. The ability to derive melanocytes from induced pluripotent stem cells (iPSCs) offers a powerful platform for disease modeling, regenerative medicine, drug screening, and personalized dermatology.
Creative Biolabs specializes in the robust and reproducible generation of melanocytes from iPSCs using a stepwise, feeder-free, chemically defined protocol that ensures high efficiency and clinical relevance.
During embryonic development, melanocytes originate from the neural crest, a transient and highly migratory cell population derived from the ectoderm. Under specific signaling cues—namely Wnt, BMP, and endothelin pathways—neural crest cells (NCCs) are induced to form melanoblasts, which then differentiate into mature, melanin-producing melanocytes.
By mimicking this ontogeny in vitro, researchers can steer iPSCs through sequential lineage commitment.
Each phase is governed by a precise balance of signaling molecules and growth factors, including dual SMAD inhibition to promote neural induction, Wnt activation to induce NCCs, and cytokines such as SCF and EDN3 to direct melanogenic lineage commitment. This stepwise process allows high-fidelity modeling of melanocyte development in vitro.
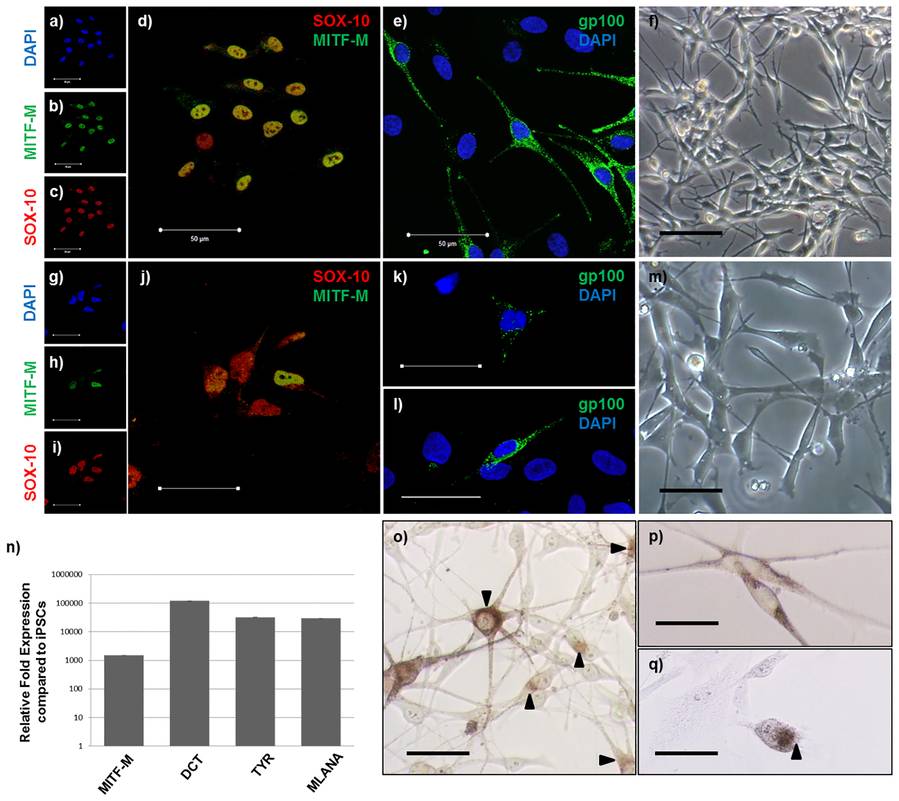 Fig.1 iPSC-derived melanocytes express normal melanocyte markers and produce melanin.1,2
Fig.1 iPSC-derived melanocytes express normal melanocyte markers and produce melanin.1,2
Creative Biolabs has optimized the process to ensure high differentiation efficiency, minimal batch-to-batch variability, and compatibility with downstream applications such as transcriptomics, proteomics, CRISPR gene editing, and compound screening.
| Reagent | Purpose |
|---|---|
| mTeSR1 or Essential 8 | Maintenance of undifferentiated iPSCs |
| N2 and B27 supplements | Neural induction media components |
| DMEM/F12, Neurobasal medium | Basal medium for different stages |
| SB431542, LDN193189 | Dual SMAD inhibition for ectoderm induction |
| CHIR99021 | Wnt pathway activation |
| BMP4, SCF, EDN3, FGF2, bFGF | Melanocyte lineage commitment |
| cAMP analog (e.g., forskolin) | Promotes melanogenesis |
| Tyrosine, PMA | Stimulates melanin biosynthesis |
| ROCK inhibitor | Enhances survival post-dissociation |
Ensure iPSCs are ~70–80% confluent and free of spontaneous differentiation. Pre-coat plates with Matrigel or recombinant vitronectin overnight. Plate iPSCs at optimal density in mTeSR1 + Y-27632 to enhance survival. Regularly monitor morphology and use live-cell markers (e.g., TRA-1-60) to confirm pluripotency before initiating differentiation.

Medium composition: DMEM/F12 + N2 + B27 (no Vitamin A). Add SB431542 + LDN193189 for dual SMAD inhibition. CHIR99021 for Wnt activation. BMP4 to modulate ectodermal patterning. Replace media daily. Maintain cells for 6–7 days until spindle-shaped neural crest-like cells emerge.

Medium switch: Neurobasal + DMEM/F12 + N2/B27 + FGF2. Add EDN3, SCF, BMP4. Maintain for another 5–7 days. Observe pigmentation in colonies and increased dendritic morphology.

Medium composition: Melanocyte growth medium (e.g., M254 with HMGS or custom melanogenic media). Supplements: SCF, EDN3, FGF2, cAMP analog (e.g., forskolin), tyrosine.
| Parameter | Methodology | Expected Outcome |
|---|---|---|
| Neural Crest Markers | IF / Flow: SOX10, PAX3 | >85% positivity at Day 7 |
| Melanocyte Lineage Markers | IF / qPCR: MITF, TYR, DCT | Robust expression by Day 14–21 |
| Melanin Production | Fontana-Masson staining / Abs at 405 nm | Progressive increase over time |
| Functional Testing | UV-induced response, pigmentation assays | Functional morphology and response |
| Purity Assessment | Flow cytometry for HMB-45, TYRP1 | >80% purity recommended |
Achieving high-efficiency and reproducible melanocyte differentiation from iPSCs requires strict control over cell state, signaling pathway activation, and timing of media changes. Below is a comprehensive guide to common troubleshooting scenarios, their underlying causes, and practical optimization strategies.
| Problem | Possible Cause | Solution |
|---|---|---|
| Neural crest induction failure |
|
|
| Low cell viability post-dissociation |
|
|
| Poor melanoblast commitment |
|
|
| Heterogeneous cell populations |
|
|
| No pigmentation |
|
|
| Senescence during expansion |
|
|
At Creative Biolabs, we proactively address these challenges by continuously optimizing protocols, validating every batch of reagents, and leveraging our in-house quality control expertise. For clients with custom differentiation needs or unique line sensitivities, we also offer protocol adjustment and pilot-scale feasibility studies.
Need help troubleshooting your iPSC-to-melanocyte workflow? Contact our expert team for one-on-one technical support.
Our offerings are modular, customizable, and backed by over two decades of stem cell innovation.
Generation of integration-free iPSC lines from PBMCs, fibroblasts, or other somatic sources using Sendai virus, episomal vectors, or mRNA.
CRISPR/Cas9-mediated knock-in, knock-out, or correction of disease-associated mutations in iPSCs; includes clonal selection and genotyping.
Pluripotency verification (OCT4, NANOG, TRA-1-81), karyotyping, trilineage differentiation, mycoplasma testing.
Differentiation of iPSCs into peripheral neurons, Schwann cells, enteric neurons, and melanocytes.
For customized projects or consultation, please contact our stem cell experts.
References
Created July 2025
For Research Use Only. Not For Clinical Use.