Dendritic cells (DCs) are the most potent antigen-presenting cells in the immune system, playing a central role in T cell activation, immune modulation, and tolerance. However, the limited availability and heterogeneity of primary DCs have hindered standardized immunological studies and clinical applications. Induced pluripotent stem cells (iPSCs) offer an unlimited and renewable source to generate homogeneous populations of functional DCs. Creative Biolabs has optimized a robust and reproducible protocol for the generation of functional dendritic cells from human iPSCs.
Conventional methods for obtaining DCs—primarily from peripheral blood mononuclear cells (PBMCs) or bone marrow—are limited by donor variability, ethical constraints, and the inability to expand cells indefinitely. These limitations pose substantial challenges for basic immunological studies and translational applications.
Human iPSCs offer an unlimited, renewable source of autologous or allogeneic immune cells with defined genetic backgrounds. Differentiating iPSCs into DCs not only ensures scalability and reproducibility but also allows for precise genetic engineering to study immune regulation, antigen presentation, and immune tolerance in both healthy and diseased contexts.
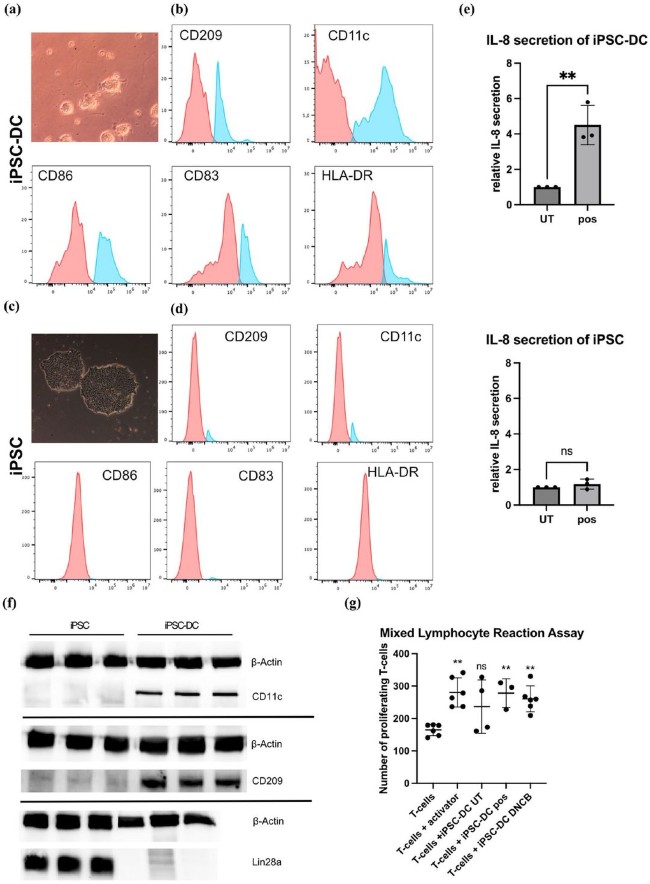 Fig.1 Functional and molecular characterization of iPSC-DC.1,2
Fig.1 Functional and molecular characterization of iPSC-DC.1,2
Creative Biolabs has established a reliable and streamlined protocol for generating high-purity, functionally competent DCs from human iPSCs.
| Reagent | Specification |
|---|---|
| iPSC Line | Integration-free, validated for pluripotency |
| Matrix | hESC/iPSC-qualified extracellular matrix |
| Medium | Xeno-free, feeder-independent, basal medium for immune cell culture |
| Gentle Cell Dissociation Reagent | For passaging iPSCs |
| Hematopoietic Differentiation Kit | Induces mesoderm and hematopoietic lineages |
| Cytokines and Growth Factors | GM-CSF, IL-4, SCF, FLT3-Ligand, TNF-α or LPS |
| Flow Cytometry Antibodies | CD11c, CD80, CD86, HLA-DR, CD83, CD34, CD43 |
| ELISA Kits | IL-12, IL-6, TNF-α cytokine detection |
Coat 6-well plates with Matrigel and incubate. Seed iPSCs at appropriate density in medium. Change medium daily and monitor morphology (compact colonies with clear edges). Passage when ~80% confluent.

Replace with Hematopoietic Induction medium. Culture for 3 days, changing medium daily. Confirm mesodermal induction by CD34+/CD43+ expression using flow cytometry.

Continue culturing cells in hematopoietic expansion medium. CD34+ progenitors typically peak by Day 7. Isolate CD34+ cells using magnetic beads (optional, for higher purity).

Resuspend CD34+ cells in RPMI + 10% FBS supplemented with GM-CSF and IL-4. Plate cells in 12-well plates. Change medium every 2–3 days. Cells will begin to exhibit immature dendritic morphology (veiled, dendrite-like protrusions).

To induce maturation, treat cells with LPS or TNF-α for 2 days. Mature DCs express high levels of CD80, CD83, CD86, and HLA-DR.
To ensure the successful derivation of functional DCs from iPSCs, Creative Biolabs conducts comprehensive quality control (QC) and functional characterization throughout the differentiation workflow. These assessments validate identity, purity, phenotype, and functional competence.
| Analysis | Description |
|---|---|
| Cell Morphology and Imaging |
|
| Phenotypic Analysis |
Flow cytometry: Use multicolor panels to confirm DC-specific surface markers:
|
| Functional Assays |
|
Below is a curated table of common issues encountered during iPSC-to-DC differentiation and their actionable solutions.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low CD34+ yield | Suboptimal mesoderm induction |
|
| Cell detachment or death during transition | Shear stress or medium shock |
|
| Poor DC marker expression | Insufficient IL-4/GM-CSF levels or inactive cytokines |
|
| Excess CD14+ cells | Monocytic skewing |
|
| Low T cell activation in MLR | Excessive IL-2 or culture stress |
|
| Variable cytotoxicity results | Immature DCs or incorrect co-culture ratio |
|
| Cell aggregation in late-stage culture | Over-confluency or cytokine depletion |
|
To maximize yield, functionality, and consistency of iPSC-derived DCs, we recommend the following expert-level refinements.
Creative Biolabs offers a comprehensive suite of services supporting dendritic cell research and iPSC technologies.
iPSC-derived DCs offer a high-fidelity, scalable model to investigate innate and adaptive immune mechanisms. Their reproducibility and accessibility significantly advance immunological research and drug development. At Creative Biolabs, our iPSC-to-DC protocol ensures efficiency, purity, and functional performance, providing reliable tools for cutting-edge immunological studies.
References
For Research Use Only. Not For Clinical Use.