Schwann cells are the principal glial cells of the peripheral nervous system (PNS), responsible for axonal myelination and trophic support. As a critical component of nerve regeneration studies, disease modeling, and cell therapy, Schwann cells derived from induced pluripotent stem cells (iPSCs) provide an unlimited, patient-specific, and ethically acceptable cell source. Creative Biolabs offers state-of-the-art custom iPSC differentiation services, enabling researchers to obtain functionally mature Schwann cells with high purity and consistency.
This protocol outlines a step-by-step strategy to generate Schwann cells from iPSCs using a staged differentiation approach that mimics embryonic development, transitioning through neural crest intermediates. The methodology is optimized for reproducibility, scalability, and downstream applications in drug screening, disease modeling, and regenerative medicine.
The generation of Schwann cells from human iPSCs is a multi-stage, lineage-guided differentiation process that closely recapitulates peripheral nervous system development in vivo. Schwann cells originate from neural crest cells (NCCs), a transient multipotent cell population arising during embryogenesis. Thus, to faithfully mimic this developmental trajectory, iPSCs must be induced through a neural lineage, transitioned into NCCs, and finally matured into Schwann cells using specific biochemical cues.
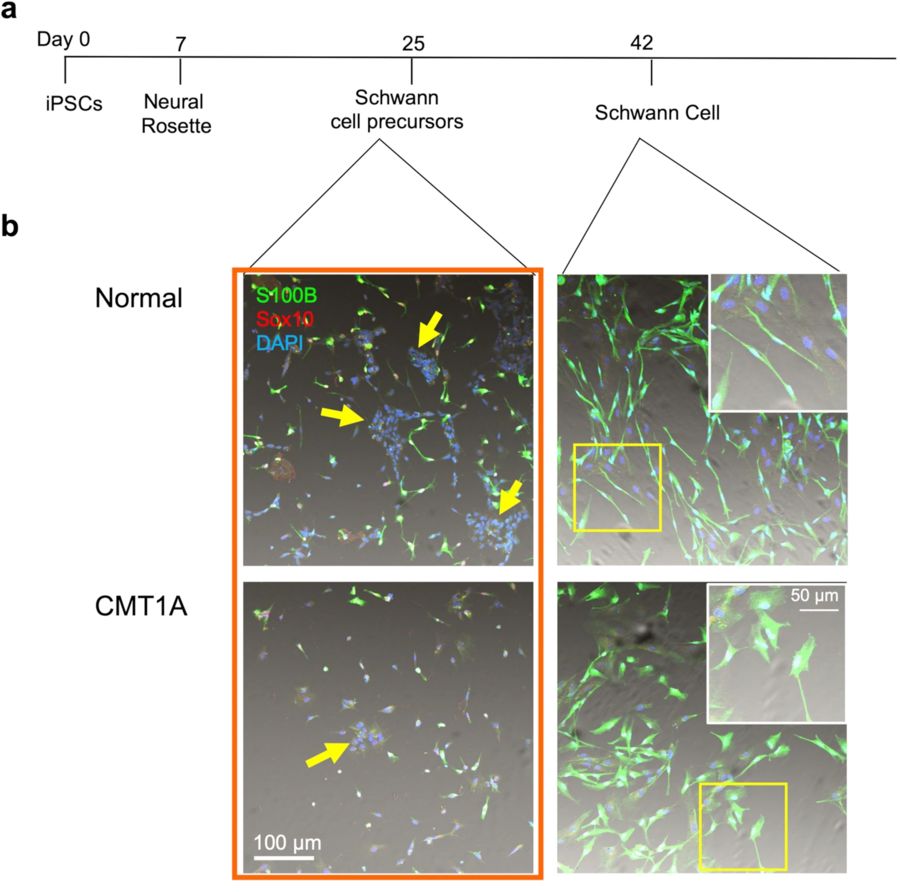 Fig. 1 Protocol for Schwann cell differentiation from iPSCs.1,2
Fig. 1 Protocol for Schwann cell differentiation from iPSCs.1,2
This process yields functionally relevant Schwann cells expressing lineage-specific markers (S100β, MBP, GFAP) and displaying key physiological behaviors such as myelination and neurotrophic support.
Importantly, iPSC-derived Schwann cells offer several advantages over primary cells.
By leveraging Creative Biolabs' deep expertise in stem cell biology and neural differentiation, researchers gain access to a powerful platform for modeling peripheral neuropathies, screening neuroprotective compounds, and advancing cell-based therapies.
| Category | Item Description |
|---|---|
| iPSC culture | Matrigel, mTeSR1 medium, Accutase |
| Induction medium | Neurobasal medium, DMEM/F12, N2 supplement, B27 supplement |
| Signaling modulators | CHIR99021 (Wnt activator), SB431542 (TGF-β inhibitor), RA |
| Schwann differentiation | Heregulin-1β (NRG1), forskolin, PDGF-BB, IGF-1, BDNF |
| Coating materials | Poly-L-ornithine (PLO), laminin |
| Antibodies (validation) | S100β, p75NTR, SOX10, GFAP, MBP |
| Others | Penicillin/Streptomycin, PBS, Trypan blue, FBS |
Culture iPSCs on Matrigel-coated plates using mTeSR1. Passage every 5–6 days using Accutase. Ensure 70–80% confluency and healthy colonies before initiating differentiation.

Replace mTeSR1 with neural induction medium: DMEM/F12 + N2 + B27 + CHIR99021 + SB431542. Feed cells daily and monitor morphology—neuroepithelial rosettes should appear around Day 5. Optional: Apply dual-SMAD inhibition (LDN193189 may be added) for enhanced neural lineage commitment.

Transition to NCC induction medium: Neurobasal + N2 + B27 + CHIR99021 + BMP4. Add Wnt3a if enhanced efficiency is desired. NCC markers (SOX10, p75NTR) should be detectable around Day 10. Use gentle enzymatic dissociation for passaging to avoid loss of fragile NCCs.

Transfer NCCs to PLO/laminin-coated plates. Switch to Schwann induction medium: DMEM/F12 + N2 + B27 + Heregulin-1β, forskolin, IGF-, BDNF, and PDGF-BB. Change medium every 2 days. By Day 30+, observe elongated, bipolar Schwann cell-like morphology. Gradually reduce forskolin to avoid overproliferation.
| Marker | Description |
|---|---|
| S100β | Schwann cell marker |
| p75NTR | Neural crest & immature Schwann cell |
| SOX10 | Neural crest lineage marker |
| MBP | Myelin basic protein (mature cells) |
| GFAP | Intermediate filament protein |
Efficient and reproducible generation of Schwann cells from iPSCs requires precise control of culture conditions, timing of factor exposure, and regular monitoring of cell health. Below is an expanded troubleshooting guide to help resolve common challenges and optimize outcomes across each stage of the protocol.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low efficiency of neural induction |
|
|
| Neural crest markers not detected |
|
|
| Excessive cell death after neural crest induction |
|
|
| Poor Schwann cell morphology |
|
|
| Low expression of S100β or MBP |
|
|
| Poor cell attachment after plating |
|
|
| Contamination or morphological abnormalities |
|
|
Creative Biolabs provides a comprehensive portfolio of customized services to support the entire workflow of Schwann cell generation from iPSCs.
Creative Biolabs is your ideal partner, providing custom iPSC services and functional validation platforms to support every step of your research journey.
Learn more or request a custom quote.
References
Created July 2025
For Research Use Only. Not For Clinical Use.