Retroviral delivery remains a dependable, cost-efficient route for generating iPSCs, especially from mouse fibroblasts and robust human donor cells. Compared with non-integrating systems, retroviruses typically yield higher colony numbers at lower COGs, and the workflow is familiar to most cell labs.
Creative Biolabs builds protocols that cover retrovirus-mediated reprogramming of somatic cells into iPSCs using integrating retroviral vectors. If speed, robustness, and budget matter, this protocol earns its place on your bench.
Retrovirus‐mediated reprogramming is a classic, high-performance route to generate iPSCs by enforcing transient, high-level expression of pluripotency factors in somatic cells. In its standard form, replication-defective Moloney murine leukemia virus vectors carry OCT4, SOX2, KLF4, and c-MYC (OSKM) as individual or polycistronic cassettes. Following transduction of dividing target cells, the vectors integrate into the host genome, initiating a cascade of epigenetic remodeling that resets the somatic program toward a stable, self-renewing pluripotent state.
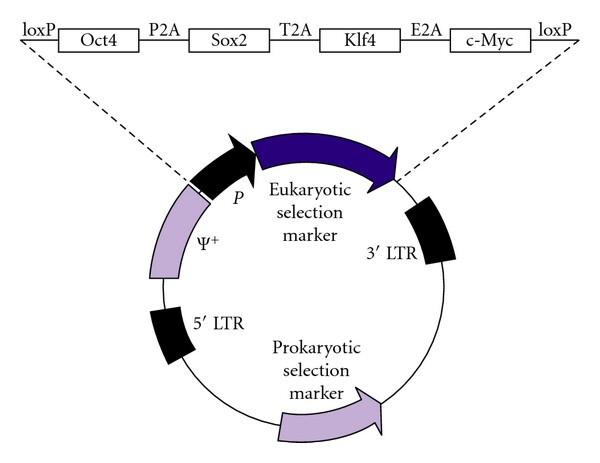 Fig.1 Retroviral reprogramming vector.1,2
Fig.1 Retroviral reprogramming vector.1,2
| Category | Reagents |
|---|---|
| Somatic cells | Human fibroblasts, PBMCs, or keratinocytes |
| Retroviral vectors | OCT4, SOX2, KLF4, c-MYC (individually or polycistronic) |
| Packaging cell line | HEK293T or phoenix cells |
| Packaging plasmids | Gag-Pol, Env, Rev |
| Culture media | DMEM/F12, KnockOut serum replacement, bFGF |
| Polymer enhancers | Polybrene to facilitate infection |
| Coating substrates | Matrigel, vitronectin, or laminin for feeder-free conditions |
| Reagents for QC | Antibodies against OCT4, NANOG, SSEA-4, TRA-1-60 |
Transfect HEK293T packaging cells with retroviral vector plasmids and helper plasmids. Collect viral supernatant 48–72 hours post-transfection. Filter through filter and concentrate virus by ultracentrifugation if needed.

Plate somatic cells at ~50% confluence one day before infection. Add retroviral supernatant with polybrene. Incubate 24 hours, then repeat infection the next day for maximum efficiency. Replace with fresh culture medium.

Maintain cells in fibroblast medium until signs of reprogramming appear. Change medium daily to remove residual virus. Monitor for reduced proliferation lag.

Switch to feeder-free iPSC medium supplemented with bFGF. Replace media every day. Observe for morphological changes.

Emerging colonies will appear compact with high nuclear-to-cytoplasmic ratio. Pick candidate colonies manually between days 18–25. Transfer to fresh coated plates for expansion.

Expand colonies under feeder-free, xeno-free conditions. Passage with EDTA or enzyme-free methods to maintain pluripotency. Confirm expression of pluripotency markers (OCT4, NANOG, TRA-1-60, SSEA4). Perform RT-PCR or qPCR for endogenous gene expression. Conduct karyotype stability analysis.
At Creative Biolabs, our scientific team has accumulated extensive hands-on experience to help clients identify problems early and apply effective solutions. Below we summarize common troubleshooting scenarios and optimization strategies across the entire workflow.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low viral titer or poor infectivity |
|
|
| Inefficient transduction of somatic cells |
|
|
| Cell survival post-transduction |
|
|
| Delayed or absent colony formation |
|
|
| Morphologically abnormal colonies |
|
|
We offer a comprehensive portfolio of stem cell services.
By partnering with Creative Biolabs, you gain access to 20 years of expertise, dedicated project management, and a global reputation for excellence.
A: Human fibroblasts are considered the gold standard due to their robust proliferation and high reprogramming efficiency. Other commonly used sources include PBMCs, keratinocytes, and occasionally urine-derived epithelial cells. The choice often depends on sample availability and the downstream application. Creative Biolabs provides customized reprogramming strategies tailored to each cell source.
A: Efficiency depends on starting cell quality, viral titer, multiplicity of infection (MOI), polybrene concentration, and culture conditions such as oxygen tension. Senescent or contaminated cells significantly reduce efficiency. Creative Biolabs offers optimization services, including small-molecule enhancers and feeder-free culture strategies, to help clients maximize yield and reproducibility.
A: Such colonies are often the result of partial reprogramming or stress during early culture. Researchers should select only compact, ES-like colonies with high nuclear-to-cytoplasmic ratios. At Creative Biolabs, our team provides morphology assessment and automated imaging support to help clients expand only the highest-quality colonies.
A: Absolutely. In addition to custom retroviral reprogramming, we offer pluripotency characterization, directed differentiation, genome editing, and organoid development services. This end-to-end pipeline allows researchers to move seamlessly from reprogramming to advanced applications without needing multiple service providers.
A: Colonies usually begin to emerge around 10–14 days after viral transduction, with well-defined ES-like colonies visible by 21–25 days. Establishing and validating stable iPSC lines generally requires 6–8 weeks. Creative Biolabs' optimized protocols streamline this timeline and include integrated quality control steps to ensure robust pluripotency validation.
References
Created September 2025
For Research Use Only. Not For Clinical Use.