At Creative Biolabs, we provide comprehensive tri-lineage differentiation ability analysis services tailored for MSC characterization. This protocol offers an in-depth guide, highlights our technical strengths, and presents optimized strategies to ensure robust, reproducible results for both academic and industrial clients.
Tri-lineage differentiation analysis is not optional but essential. It:
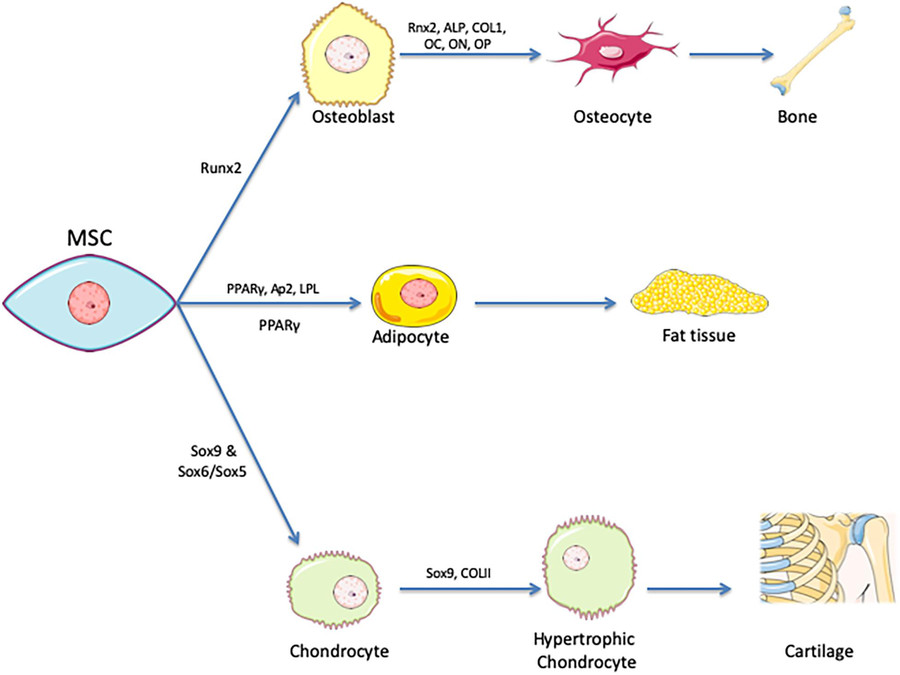 Fig.1 Tri-lineage encompasses differentiation of MSCs.1,2
Fig.1 Tri-lineage encompasses differentiation of MSCs.1,2
The principle relies on directed differentiation of MSCs using lineage-specific induction media, followed by staining or molecular confirmation of lineage-specific markers.
This three-tiered assessment provides a comprehensive functional profile of MSC multipotency.
| Category | Item |
|---|---|
| General Culture |
MSCs (human or animal source) at early passages (P2–P5 preferred) DMEM or α-MEM Fetal bovine serum (FBS), MSC-qualified Antibiotics (penicillin/streptomycin) Trypsin-EDTA solution |
| Osteogenic Differentiation |
Dexamethasone Ascorbic acid β-Glycerophosphate Alizarin Red S solution Alkaline Phosphatase (ALP) kit |
| Adipogenic Differentiation |
Insulin Dexamethasone Indomethacin IBMX Oil Red O solution |
| Chondrogenic Differentiation |
High-glucose DMEM Insulin-transferrin-selenium (ITS) Sodium pyruvate Ascorbic acid TGF-β3 Alcian Blue solution |
Seed MSCs in 6-well plates. Once 70–80% confluent, replace growth medium with osteogenic induction medium. Change medium every 3 days. After 14–21 days, fix cells with 4% paraformaldehyde. Stain with Alizarin Red S or perform ALP assay. Visualize mineralized nodules under microscope.

Seed MSCs at high density. Induce with adipogenic medium containing IBMX, dexamethasone, indomethacin, and insulin for 3 days. Switch to maintenance medium (with insulin only) for 1–2 days. Repeat induction/maintenance cycles for 2–3 weeks. Fix with paraformaldehyde and stain with Oil Red O. Assess intracellular lipid droplets microscopically.

Centrifuge MSCs to form a pellet. Add chondrogenic induction medium containing TGF-β3, ITS, ascorbic acid, and sodium pyruvate. Culture for 21 days, replacing medium every 3 days. Fix pellets, embed in paraffin, and section. Stain sections with Alcian Blue or Safranin O for proteoglycan detection. Evaluate cartilage-like matrix formation.
Below is an expanded troubleshooting and optimization guide, designed to help researchers interpret anomalies and maximize reproducibility.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low differentiation efficiency across all lineages |
|
|
| weak or no mineralization detected |
|
|
| Few or small lipid droplets observed |
|
|
| Pellet formation fails |
|
|
| Pellet core necrosis |
|
|
Tri-lineage differentiation is only one part of a comprehensive stem cell characterization pipeline. At Creative Biolabs, we provide a wide range of stem cell analysis services to ensure that your MSCs and other stem cell types meet international quality and identity standards.
A: Differentiation potential diminishes with higher passages. Typically, MSCs beyond passage 6–8 show reduced efficiency. For optimal results, we recommend using early-passage cells (P2–P5). If late-passage cells must be used, we provide tailored optimization strategies to enhance outcomes.
A: The assay generally requires 2–4 weeks, depending on the lineage. Osteogenesis often takes the longest, up to 28 days, while adipogenesis and chondrogenesis typically show clear results within 14–21 days. At Creative Biolabs, we optimize protocols to balance speed and accuracy, ensuring reliable and reproducible outcomes.
A: Donor-to-donor variability is common. To address this, we recommend analyzing multiple donors side by side. Our service includes comparative data sets, highlighting relative differentiation efficiency. This approach helps clients select the most suitable donor line for downstream applications or product development.
A: Yes. Many clients combine this service with flow cytometry for surface marker profiling, karyotype analysis, or pluripotency marker expression assays. Creative Biolabs offers integrated service packages, enabling you to achieve comprehensive MSC identity confirmation in a single streamlined workflow.
References
Created August 2025
For Research Use Only. Not For Clinical Use.