Among the many lineages that can be derived from iPSCs, chondrocytes, cartilage-producing cells, hold exceptional promise for modeling osteoarthritis, studying cartilage biology, and engineering cartilage tissue.
Creative Biolabs delivers precise, reproducible, and scalable iPSC-to-chondrocyte differentiation protocols that support both basic and translational research. The following protocol outlines a robust, feeder-free, and chemically defined approach for generating functional chondrocytes from iPSCs.
Chondrocytes are the sole cellular component of cartilage, playing a critical role in maintaining the integrity of the extracellular matrix through the production of type II collagen and proteoglycans. However, the limited proliferative capacity of native chondrocytes and the avascular nature of cartilage tissue make regeneration following injury or degeneration extremely challenging. Conventional sources such as primary chondrocytes or mesenchymal stem cells (MSCs) often suffer from donor variability, limited expansion potential, and loss of chondrogenic phenotype upon subculture.
iPSCs offer a transformative solution. These reprogrammed cells possess unlimited self-renewal capacity and pluripotency, enabling the derivation of chondrocytes in a patient-specific, renewable, and ethically viable manner.
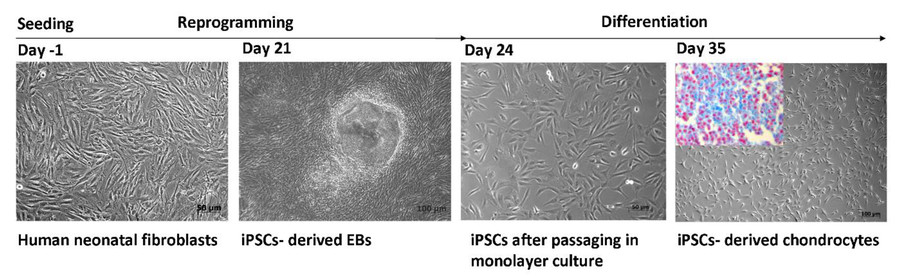 Fig.1 The generation of iPSC-derived chondrocytes through the embryoid bodies (EBs) method.1,2
Fig.1 The generation of iPSC-derived chondrocytes through the embryoid bodies (EBs) method.1,2
Nevertheless, directing iPSCs toward a stable chondrocyte phenotype requires meticulous control over differentiation signals, timing, and 3D microenvironment. An optimized and reproducible protocol is essential to overcome the heterogeneity and variability often associated with stem cell differentiation. Creative Biolabs leverages over two decades of expertise in stem cell biology to deliver standardized, scalable, and GMP-adaptable workflows for chondrocyte differentiation.
| Component | Details |
|---|---|
| iPSCs | Validated pluripotent iPSC line |
| Media |
Medium for iPSC maintenance Mesoderm induction medium (Contains Activin A, BMP4, FGF2) Chondrogenic induction medium (Contains TGF-β3, dexamethasone, ascorbic acid) |
| Coating reagents | For iPSC culture plates |
| Supplements | ITS+ Premix, Sodium Pyruvate, L-Glutamine (For chondrogenic phase) |
Maintain iPSCs in medium on Matrigel-coated plates. Passage iPSCs when colonies reach ~80% confluency. Confirm pluripotency markers (OCT4, NANOG, SOX2) via immunostaining before initiating differentiation.

Replace iPSC medium with mesoderm induction medium. Change media daily. Observe cell morphology: elongated spindle-like appearance suggests mesoderm commitment.

Transfer cells to low-attachment plates to initiate condensation. Culture as aggregates to mimic mesenchymal condensation. Replace media every other day.

Centrifuge cells in a 96-well round-bottom plate. Add chondrogenic medium. Incubate pellets. Do not disturb for first 48 h. Change media every 2–3 days. Gene expression of COL2A1, ACAN, and SOX9 indicates mature chondrocytes.
To ensure the identity, purity, functionality and safety of iPSC-derived chondrocytes, Creative Biolabs has implemented a comprehensive quality control (QC) process that ensures cell identity and lot consistency.
| Evaluation Category | Assay Type | Purpose |
|---|---|---|
| Gene Expression Profiling | qPCR and RT-PCR |
|
| Protein-Level Validation | Immunostaining & Western Blot |
|
| Extracellular Matrix Quantification | Biochemical Assays |
|
| Immunophenotyping | Flow Cytometry |
|
An efficient and consistent iPSC-to-chondrocyte differentiation process requires precise control of culture conditions. Below are common issues encountered during the workflow and strategies for resolution.
| Problem | Possible Cause | Solution |
|---|---|---|
| Poor mesoderm induction | Suboptimal cytokine activity, incorrect plating density |
|
| High cell death during early differentiation | Medium shock, shear stress during handling |
|
| Pellet not forming properly | Low cell number, low viability, inadequate aggregation |
|
| Small, loose, or fragile pellets | Incomplete condensation, poor matrix production |
|
| Low COL2A1 or SOX9 expression | Insufficient exposure to chondrogenic cues |
|
| Matrix deposition not detected | Incomplete differentiation, reagent degradation |
|
To enhance the efficiency, reproducibility, and maturity of iPSC-derived chondrocytes, consider the following optimization strategies based on our extensive in-house development experience.
At Creative Biolabs, we provide a comprehensive portfolio of iPSC-based services to support your disease modeling and cell therapy development pipelines. Whether you are at the early stage of iPSC line generation or preparing for preclinical evaluation, our custom services are designed to ensure scientific excellence, technical reliability, and regulatory compliance.
Custom iPSC generation from somatic cells (PBMCs, fibroblasts, etc.)
Mesoderm induction from iPSCs, chondrocyte differentiation optimization, other lineage-specific differentiations: osteoblasts, adipocytes, MSC-like cells.
iPSC-chondrocyte-based drug testing models
The generation of chondrocytes from iPSCs is a complex yet highly scalable process, requiring stringent control of signaling cues and culture conditions. By following this streamlined protocol and leveraging Creative Biolabs' customizable solutions, researchers can obtain high-quality chondrocytes suitable for various applications.
Partner with Creative Biolabs to accelerate your project with confidence and scientific excellence.
References
For Research Use Only. Not For Clinical Use.