In the stem cell research world, one question echoes louder than the rest: How do we know our pluripotent stem cells are truly pluripotent? Among all the assays developed to answer this, the in vivo teratoma formation assay remains the gold standard.
Researchers rely on teratoma formation to validate the quality of their induced pluripotent stem cells (iPSCs) or embryonic stem cells (ESCs), and Creative Biolabs brings two decades of experience in delivering protocols that are not only reproducible but also finely optimized for success.
The teratoma formation assay is built on a fundamental biological concept: pluripotent stem cells have the ability to differentiate into cell types representing all three embryonic germ layers. When transplanted into an appropriate in vivo microenvironment, typically an immunocompromised host, they undergo spontaneous, uncontrolled differentiation. This process leads to the development of a benign tumor-like mass known as a teratoma.
Unlike in vitro assays such as EB formation or directed differentiation, the teratoma assay provides a living, three-dimensional context in which stem cells interact with host vasculature, extracellular matrix, and physiological signaling pathways. This mimics the complexity of embryonic development more closely than any dish-based system can achieve.
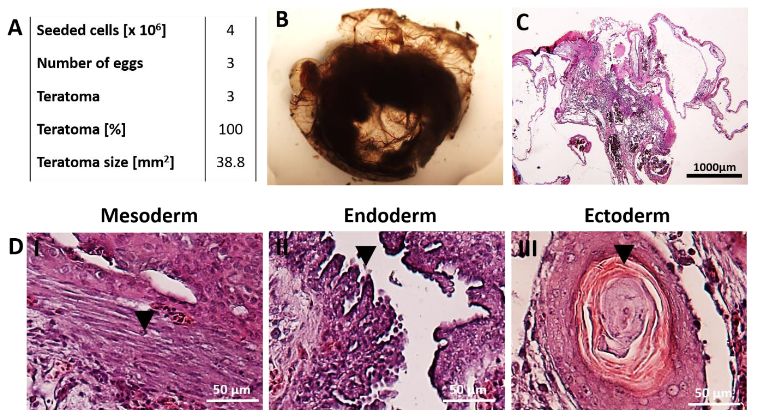 Fig.1 Generation of teratomas by seeding hiPSCs.1,2
Fig.1 Generation of teratomas by seeding hiPSCs.1,2
In essence, the teratoma assay acts as a biological litmus test for pluripotency. By taking stem cells out of the artificial constraints of culture dishes and placing them in a living system, researchers gain the most definitive evidence of their developmental capacity.
At Creative Biolabs, our protocols emphasize not just generating teratomas, but also extracting maximum information through careful histological characterization, lineage-specific marker staining, and high-resolution digital pathology.
| Category | Item |
|---|---|
| Stem Cells and Culture Media |
Pluripotent stem cells Feeder-free or xeno-free culture medium Pluripotency supplements Cryopreservation reagents |
| Animal Models |
Immunocompromised mice Sterile bedding and autoclaved water/food Animal identification tags |
| Injection-Related Reagents |
Extracellular matrix (ECM) support Physiological buffer Anesthetic agents Analgesics |
| Histology and Tissue Processing Reagents |
Fixatives Dehydration reagents Embedding medium Microtome and cryostat blades for thin tissue sectioning Hematoxylin and eosin (H&E) staining kits Lineage-specific antibodies |
Expand pluripotent stem cells under feeder-free, xeno-free conditions to maintain pluripotency. Verify cell quality via pluripotency markers (Oct4, Nanog, SSEA4). Harvest cells during logarithmic growth phase. Resuspend cells.

Use immunocompromised mice aged 6–8 weeks. Maintain mice in sterile, pathogen-free housing. Anesthetize according to institutional guidelines. Shave and disinfect the injection area.

Load cells into a fine-gauge syringe. Slowly inject into the chosen site. Gently withdraw the needle, applying slight pressure to prevent leakage. Monitor mice until recovery from anesthesia.

Observe mice weekly for teratoma growth. Palpate injection sites for nodules (typically visible by 6–12 weeks). Record tumor size with calipers. Humane endpoints must be defined to prevent animal distress.

Sacrifice animals when teratomas reach ~1.0–1.5 cm diameter. Excise teratomas carefully, avoiding rupture. Fix tissues in 10% formalin for 24–48 hours. Embed in paraffin, section at 5–10 µm. Stain with H&E and evaluate under a microscope. Confirm the presence of tissues from all three germ layers.
At Creative Biolabs, our team has accumulated over 20 years of experience refining this assay, and we share below the most common issues along with practical solutions.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low or no teratoma formation |
|
|
| Necrosis or poor tissue differentiation |
|
|
| Overgrowth of teratomas |
|
|
| Difficulty in identifying germ layers |
|
|
To support researchers at every stage, we provide a suite of complementary services designed to ensure your pluripotent stem cells are robust, stable, and ready for downstream use.
A: Immunocompromised strains such as NOD/SCID, NSG, or BALB/c nude mice are commonly used. The choice depends on availability, experimental goals, and ethical regulations. We provide guidance on selecting the most suitable host to balance efficiency and compliance.
A: Histological evaluation using H&E staining is the standard. To increase precision, we also perform immunohistochemistry (IHC) with lineage-specific markers.
A: Teratomas usually become palpable within 6–12 weeks after injection. The exact timeline depends on the cell type, injection site, and host strain. Our monitoring protocols ensure tumors are harvested at the right size to avoid necrosis and animal discomfort.
A: Because it provides the most definitive evidence that stem cells can differentiate into tissues of all three germ layers within a living system. Unlike in vitro assays, it demonstrates real physiological developmental potential in an unbiased manner.
References
Created August 2025
For Research Use Only. Not For Clinical Use.