Astrocytes are the most abundant glial cells in the central nervous system (CNS) and play crucial roles in synaptic regulation, neurovascular coupling, metabolic homeostasis, and neuroinflammation. Patient-specific astrocytes derived from iPSCs offer a powerful tool to model neurodegenerative diseases, such as ALS, Parkinson's disease, Alzheimer's disease, and schizophrenia, under genetically controlled conditions.
Creative Biolabs leverages a stage-specific, chemically defined differentiation process. This protocol ensures the generation of mature, GFAP+ astrocytes within 50–80 days, with high scalability and phenotype fidelity.
The generation of astrocytes from iPSCs is a multi-stage, tightly regulated process that mirrors key developmental transitions of the human central nervous system. This approach allows researchers to derive patient-specific, genetically matched astrocytes for modeling disease, studying cell–cell interactions, and testing pharmacological agents under controlled conditions.
Unlike traditional cell lines or primary astrocytes from post-mortem brain tissue, iPSC-derived astrocytes offer superior scalability, standardization, and disease relevance, particularly in neurodegenerative and neuroinflammatory research. Through precisely timed exposure to growth factors and small molecules, iPSCs are directed through neuroectodermal induction, neural progenitor expansion, glial commitment, and ultimately astrocyte maturation.
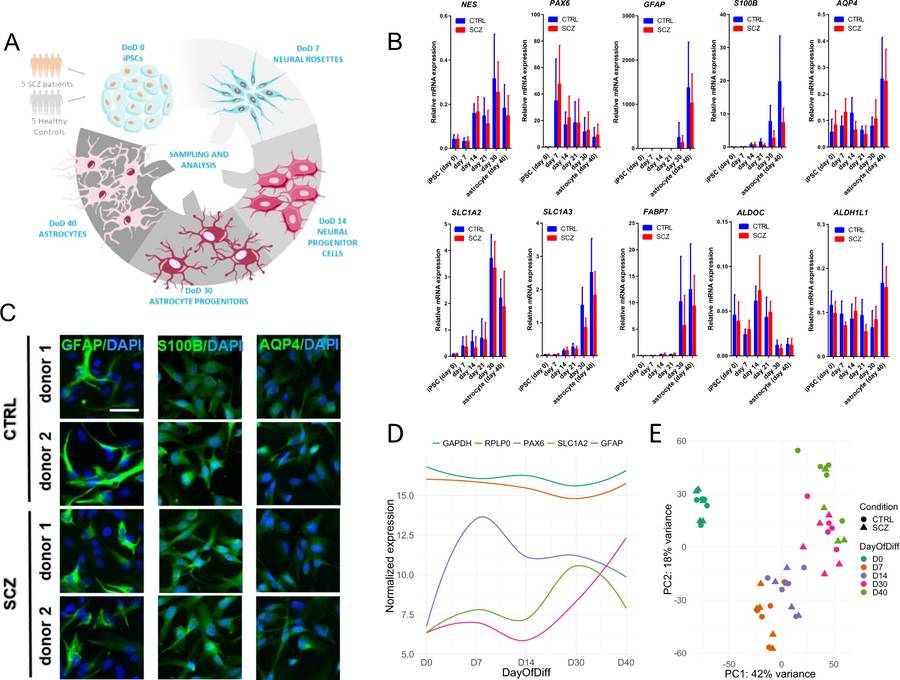 Fig.1 Characterization of iPSC-derived astrocytes.1,2
Fig.1 Characterization of iPSC-derived astrocytes.1,2
At Creative Biolabs, we adopt a developmentally inspired, chemically defined, and xeno-free differentiation protocol that ensures:
| Category | Reagents |
|---|---|
| Cell culture | Matrigel, mTeSR1, DMEM/F12, neurobasal medium |
| Small molecules | SB431542, LDN193189, CHIR99021, DAPT, BMP4 |
| Growth factors | FGF2, EGF, CNTF, BDNF, NT-3 |
| Supplements | N2, B27 (with/without vitamin A), GlutaMAX |
| Coatings | Poly-L-ornithine, Laminin |
| iPSC characterization | OCT4, NANOG, TRA-1-60 antibodies |
| Astrocyte markers | GFAP, S100β, AQP4 antibodies |
Plate iPSCs on Matrigel-coated 6-well plates at ~50% confluency in mTeSR1 medium. Perform immunostaining for pluripotency markers (OCT4, SOX2, TRA-1-60) and remove any differentiated colonies. Expand until colonies reach 70–80% confluency.

Replace mTeSR with neural induction medium: DMEM/F12 + N2 Supplement + SB431542 + LDN193189 + CHIR99021. Refresh medium daily. Neural rosettes emerge, are manually isolated and replated onto new Poly-L-ornithine/laminin-coated plates.

Medium: DMEM/F12 + N2 + B27 without vitamin A + FGF2 + EGF. Pass cells every 5–6 days when confluency exceeds 80%. Confirm neural progenitor cell identity via Nestin and PAX6 immunostaining.

Medium: Neurobasal + GlutaMAX + B27 + BMP4 + CNTF. Add DAPT to inhibit Notch signaling, favoring astroglial over oligodendroglial differentiation. Change medium every other day.

Medium: DMEM/F12 + N2 + B27 + CNTF + BDNF + NT-3. Culture for ≥4 weeks for mature astrocyte marker expression. Cells can be cryopreserved, replated for co-culture, or analyzed directly.
At Creative Biolabs, every batch of iPSC-derived astrocytes undergoes strict quality control, including morphological QC, molecular QC, purity QC, viability, mycoplasma and sterility testing. In addition, we assess astrocyte identity by immunofluorescence, flow cytometry, and qPCR using a set of typical and functional markers.
| Marker | Type | Function/Significance | Expression Level |
|---|---|---|---|
| GFAP | Intermediate filament | Gold-standard astrocyte marker; structural support | +++ |
| S100β | Calcium-binding protein | Indicator of astrocyte lineage and maturation | ++ |
| AQP4 | Membrane channel | Water transport across BBB; linked to neuroinflammation | ++ |
| ALDH1L1 | Cytosolic enzyme | Pan-astrocyte marker, present in both fetal and adult astrocytes | ++ |
| GLAST (SLC1A3) | Transporter | Regulates glutamate clearance at synapses | + |
| CD44 | Surface glycoprotein | Associated with reactive astrocytes and glial scarring | + |
Beyond morphology and marker expression, we evaluate the functional competence of astrocytes through a battery of performance assays, including:
Generating functional astrocytes from iPSCs is a complex, multi-step process that can be influenced by subtle changes in reagents, culture conditions, or handling techniques. We proactively address potential bottlenecks and provide technical guidance to help clients achieve optimal differentiation outcomes.
| Problem | Possible Cause | Solution |
|---|---|---|
| Low NPC yield | Inefficient neural induction; poor iPSC quality |
|
| Heterogeneous morphology | Incomplete lineage commitment or contamination |
|
| Delayed astrocyte maturation | Suboptimal cytokine timing or concentrations |
|
| Cell death during passage | Harsh dissociation method or poor matrix support |
|
| Low GFAP positivity | Insufficient glial commitment |
|
| Batch-to-batch variability | iPSC line variability or inconsistent media |
|
If you encounter unexpected differentiation outcomes or require assistance customizing the protocol for a specific application, our scientific team is ready to help.
With over two decades of excellence in stem cell biology and neurobiology, Creative Biolabs brings unmatched expertise to your astrocyte differentiation projects and integrated services to support every stage of your project.
Generation of high-quality human iPSCs from somatic cells using non-integrative methods.
Production of functionally mature glutamatergic, dopaminergic, GABAergic, or motor neurons from human iPSCs. Compatible with astrocyte co-culture.
Gene knockout, knock-in, or correction services for iPSC lines using precise strategies with clone validation.
Interested in integrating astrocyte models into your neurobiology workflow? Let our experts help you build a customized in vitro system tailored to your research questions.
References
Created July 2025
For Research Use Only. Not For Clinical Use.