Creative Biolabs is a contract research organization (CRO) launched and operated by a group of experts with extensive expertise and experience in cell therapy development and research. We are determined to providing innovative products and services to clients in the educational and business sectors around the world, facilitating the exploration of breakthrough scientific discoveries and actively driving the development of preclinical and clinical research.
C-mesenchymal-epithelial transition factor (c-Met), also named hepatocyte growth factor receptor (HGFR), is a member of the receptor tyrosine kinase family of proteins and the product of the proto-oncogene MET. The preproprotein is encoded and undergoes proteolytic processing to produce alpha and beta subunits. These subunits are then linked together through disulfide bonds, resulting in the formation of the mature receptor. The beta subunit undergoes further processing, leading to the creation of the M10 peptide.
The c-Met structure consists of a heterodimer composed of an alpha chain (50 kDa) and a beta chain (145 kDa), which are connected by disulfide bonds as shown in Figure 1. In its inactive state, the C-terminal tail interacts with the catalytic domain, inhibiting kinase activity. However, upon ligand binding, this interaction is disrupted, and phosphorylation occurs on the C-terminal tail, thereby increasing kinase activity.
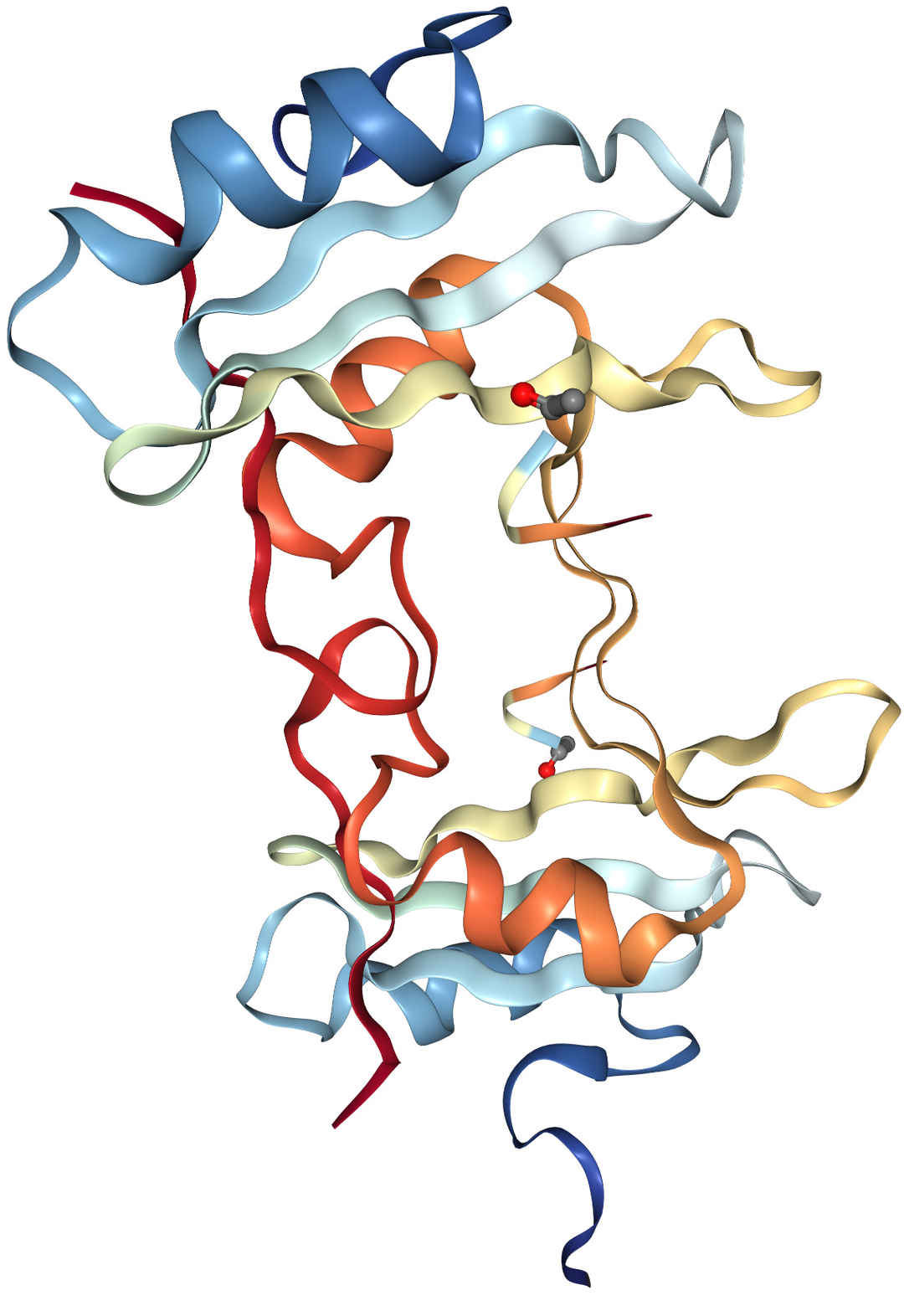 Fig.1 Structure of c-Met
Fig.1 Structure of c-Met
C-Met is expressed in cytoplasm and membrane in most tissues with a high level of protein expression in cerebral cortex, lung, duodenum, small intestine, rectum, gallbladder, cervix, and soft tissue. And overexpression exists in a range of tumor cell lines, mainly solid tumors. c was highly expressed in a range of tumor cell lines, mainly solid tumors. From the data in Proteinatlas, it is relatively more highly expressed in gastric and kidney cancer and has a very low tumor specificity. Besides, c-Met serves as a prognostic marker in pancreatic cancer (unfavorable) and head and neck cancer (unfavorable).
C-Met is a protein that transmits signals from the extracellular matrix to the cytoplasm by binding with hepatocyte growth factor (HGF) ligand. When HGF binds to c-Met on the cell surface, it triggers autophosphorylation of MET in its intracellular domain, creating docking sites for downstream signaling molecules.
Once activated by HGF, c-Met interacts with several downstream effectors such as PIK3R1, PLCG1, SRC, GRB2, STAT3 or GAB1. This recruitment leads to activation of various signaling cascades including RAS-ERK, PI3 kinase-AKT and PLCgamma-PKC. The RAS-ERK pathway is associated with morphogenetic effects while PI3K-AKT coordinates pro-survival effects. Therefore, c-Met plays a crucial role in regulating many physiological processes such as proliferation, scattering and morphogenesis which are important for both normal tissue function and tumor progression.
Mutations in Met can lead to abnormal activation of the Met signaling pathway, which can cause tumorigenesis and progression. Studies have shown that mutation and amplification in exon 14 of the Met gene occur at a high rate in non-small cell lung cancer and are the initiating factors for its tumorigenesis.
By far the FDA-approved chimeric antigen receptors (CARs) cells therapy are all for treatment of hematologic malignancies, none of which for solid tumors to date. On account of the finding that c-Met is widely expressed, mutated, and amplificated in most solid organs, c-Met may be a potential target for the application of CARs in solid cancer.
There are 3 clinical trials for 3 cancers: breast cancer, melanoma and hepatocellular carcinoma (HCC). The one for HCC used single-chain bispecific c-Met/PD-L1 CAR-T cells which has been proven to enhance therapeutic effects in animal xenograft models.
One clinical trial demonstrated the safety and efficacy of intratumoral injections of mRNA-transfected c-Met CAR-T cells in metastatic breast cancer (NCT01837602).
Introducing the CAR via mRNA ensured the limitation of the non-tumor cells targeting c-Met. Patients with accessible cutaneous or lymph node metastases received a single intratumoral injection of 3 × 107 or 3 × 108 cells. Great tolerance was shown and the CAR-T mRNA was detected in tumor tissues and peripheral blood, suggesting an evocation of inflammatory response within tumors.
Table 1. Ongoing c-Met-Targeted CAR Cell Therapy Clinical Trials
| NCT Number | Title | Status | Conditions | Phases | Sponsor/Collaborators |
| NCT03672305 | Clinical Study on the Efficacy and Safety of c-Met/PD-L1 CAR-T Cell Injection in the Treatment of HCC | Unknown status | Primary Hepatocellular Carcinoma | Early Phase 1 | The Second Hospital of Nanjing Medical University |
| NCT03060356 | Autologous T Cells Expressing MET scFv CAR (RNA CART-cMET) | Terminated |
Malignant Melanoma Breast Cancer |
Early Phase 1 | University of Pennsylvania |
| NCT01837602 | cMet CAR RNA T Cells Targeting Breast Cancer | Completed |
Metastatic Breast Cancer Triple Negative Breast Cancer |
Phase 1 | University of Pennsylvania |
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION