Quantitatively measure surface CAR expression to confirm construct presence and density.
As a pivotal innovation in immuno-oncology, CAR-Macrophages (CAR-MA) exploit the favorable tumor-homing properties and multifaceted effector responses of macrophages to circumvent key limitations of established cell therapies. Creative Biolabs' CAR-MA Assay Services offer comprehensive functional evaluation of CAR-MA products. Our platform synthesizes cutting-edge approaches, from high-resolution imaging and antigen-specific functional screens to kinetic effector response monitoring, to provide quantitative, high-fidelity data. This enables us to partner with you in navigating the path from vector design and functional validation through to successful preclinical translation.
CAR-MA are a transformative cell therapy for solid tumors, merging innate and adaptive immune properties to remodel the immunosuppressive microenvironment. Their multi-mechanistic action, encompassing potent phagocytosis, pro-inflammatory polarization, T cell priming via cross-presentation, and matrix degradation, enables a coordinated assault on cancer. Consequently, a precise, standardized, and high-throughput CAR-MA functional evaluation system serves as a critical bridge connecting fundamental research, process optimization, and clinical application.
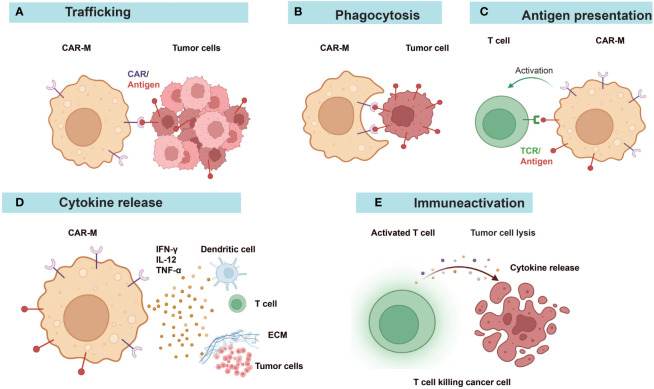 Fig.1 Diverse antitumor mechanisms of CAR-Macrophage therapy.1
Fig.1 Diverse antitumor mechanisms of CAR-Macrophage therapy.1
Leveraging the innate tumor-infiltrating ability and diverse cytotoxic mechanisms of macrophages, we engineer advanced CAR-MA strategies. To support these efforts, Creative Biolabs' CAR-MA Assay Services provide a diverse suite of assays that generate reliable data on critical functions, from phagocytosis and polarization to antigen presentation and stability, enabling informed decisions to reduce development risks and streamline the path to the clinic.
We offer a comprehensive suite of specialized assays to systematically evaluate the function, specificity, and cellular mechanisms of CAR-MA therapies, supporting their entire development workflow.
Quantitatively measure surface CAR expression to confirm construct presence and density.
Validate functional CAR engagement by assessing phagocytosis of ligand-coated silica beads.
Determine antigen specificity and phagocytic preference using customizable antigen pools and size-variable beads.
Evaluate whole-cell phagocytosis efficacy against target cells expressing endogenous antigens.
Visualize and document cellular processes in real time using high-resolution rotating disk confocal microscopy.
Measure bulk phagocytic capacity following macrophage activation.
Score individual internalization events in live cells based on high-content imaging.
Profile key cytokine secretion using standardized platforms like intracellular staining.
Characterize macrophage polarization states using multi-parameter flow cytometry, ELISA, or qPCR.
Assess the ability of CAR-MA to process and present tumor antigens to activate T-cells.
Investigate metabolic adaptations linked to effector functions through metabolomics and enzymatic assays.
Required starting materials:
Key Steps Involved:
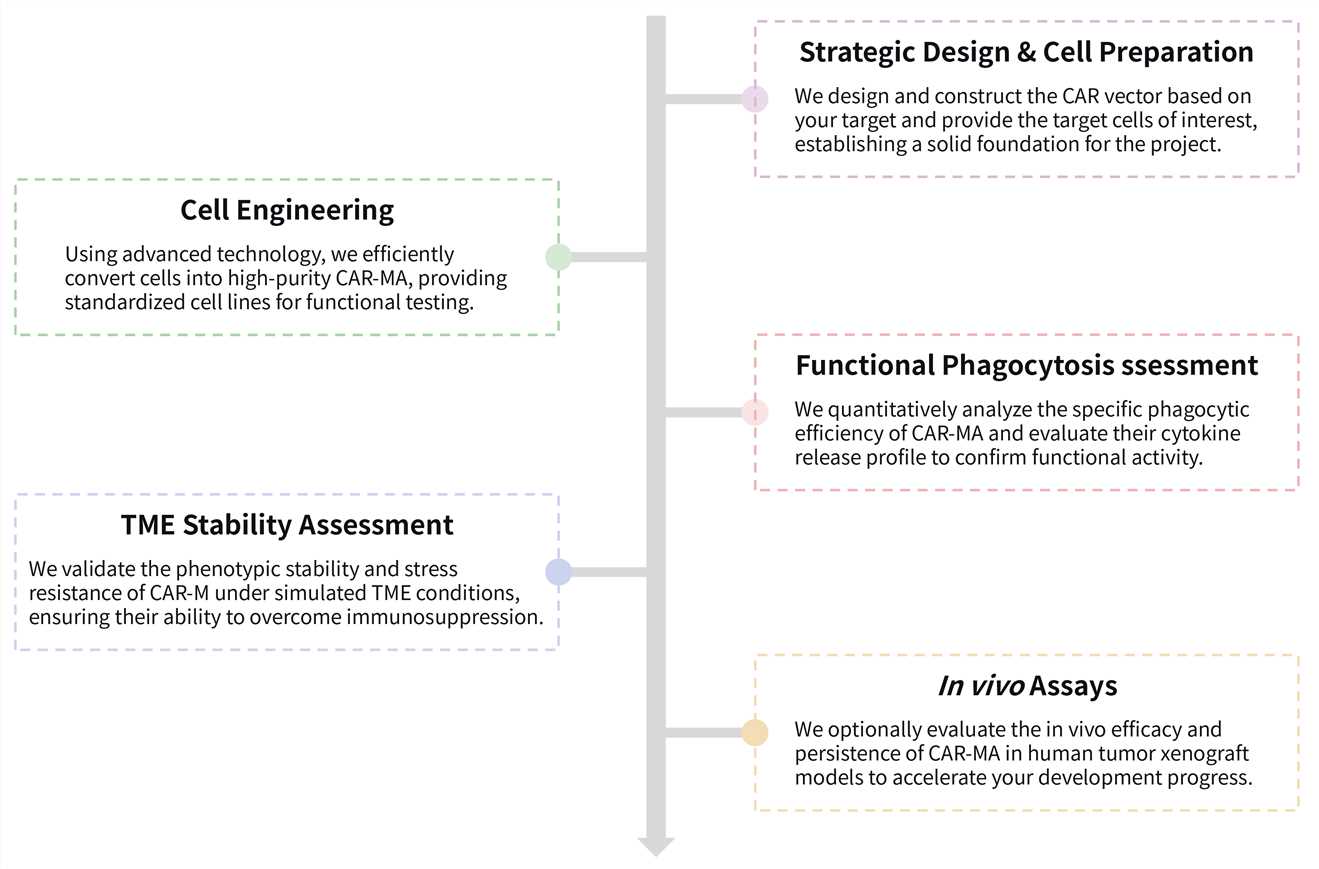
Final Deliverables:
Our support for CAR-MA projects is premised on exceptional technical knowledge and dedicated infrastructure. We ensure precise and robust outcomes through a commitment to customized, goal-oriented service.
What specific data on toxicity or off-target effects do your CAR-MA assays provide?
We provide critical in vitro safety data, including detailed cytokine release profiles and off-target phagocytosis screening against relevant non-tumor cell lines. This helps predict potential systemic side effects early in development, allowing for construct refinement before expensive in vivo or clinical studies.
Can your services help us ensure the CAR-MA phenotype remains stable (M1-like) in the tumor microenvironment?
Absolutely. Our specialized TME stability and reprogramming assays monitor M1/M2 polarization markers when your CAR-MA is challenged by immunosuppressive cues. We use our expertise in inducible gene expression systems to help design and validate constructs that resist the TME's pathological influences, ensuring durable anti-tumor activity.
We serve as your strategic partner in advancing CAR-MA therapy. We assist you in navigating the selection of optimal CAR constructs and provide a comprehensive suite of CAR-MA assay services. Our goal is to deliver reliable and actionable data that accelerates your entire research and development pipeline.
"Using Creative Biolabs' CAR-MA Assay Services in our research has significantly improved the in vivo stability modeling of our lead candidate. Their TME simulation assay accurately predicted and helped us mitigate the M2 shift that plagued our initial constructs. This saved us months of preclinical iteration."— Dr. Ke Js.
"The scalable, high-yield iPSC-to-CAR-MA protocol provided by Creative Biolabs is a game-changer for our manufacturing strategy. The resulting macrophages are functionally superior and eliminate the high cost and variability associated with primary monocyte sourcing, validating our path to an off-the-shelf product."— S. Pl Vs.
"We relied on Creative Biolabs' Phagocytosis-Optimized CAR Design consultation to refine our signaling domain. The resulting construct achieved a 65% increase in target cell engulfment compared to our control, proving the necessity of their specialized Fc-receptor derived signaling expertise. "—An Tr.
Harnessing the power of CAR-Macrophages for solid tumors is the next frontier in immuno-oncology. Let's pioneer this future together. Creative Biolabs is your partner in translating groundbreaking science into life-changing clinical solutions.
Connect with our team to start the conversation.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION