The ideal CAR-T evaluation models are the combination of mice hosting both the human immune system and patient derived tumors. These novel models called "humanized patient-derived xenograft (PDX) model", expressing a human immune system, possess a distinct advantage over other PDX in immunodeficiency mice. Engrafting these humanized mice with human tumors is the future of the preclinical therapeutic development of CAR-T treatment evaluation.
The most useful models for CAR-T research include:
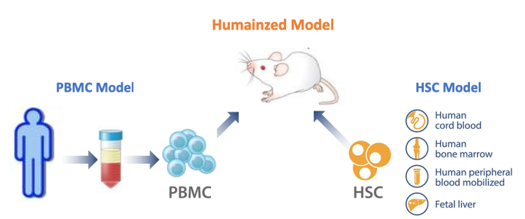
To use humanized mice effectively and ensure that you use the correct model to answer your specific questions, you need to fully understand the existence and potency of different human immune cell populations in each model. The figure below summarizes the key differences between hPBMC and hCD34 humanized mouse models:
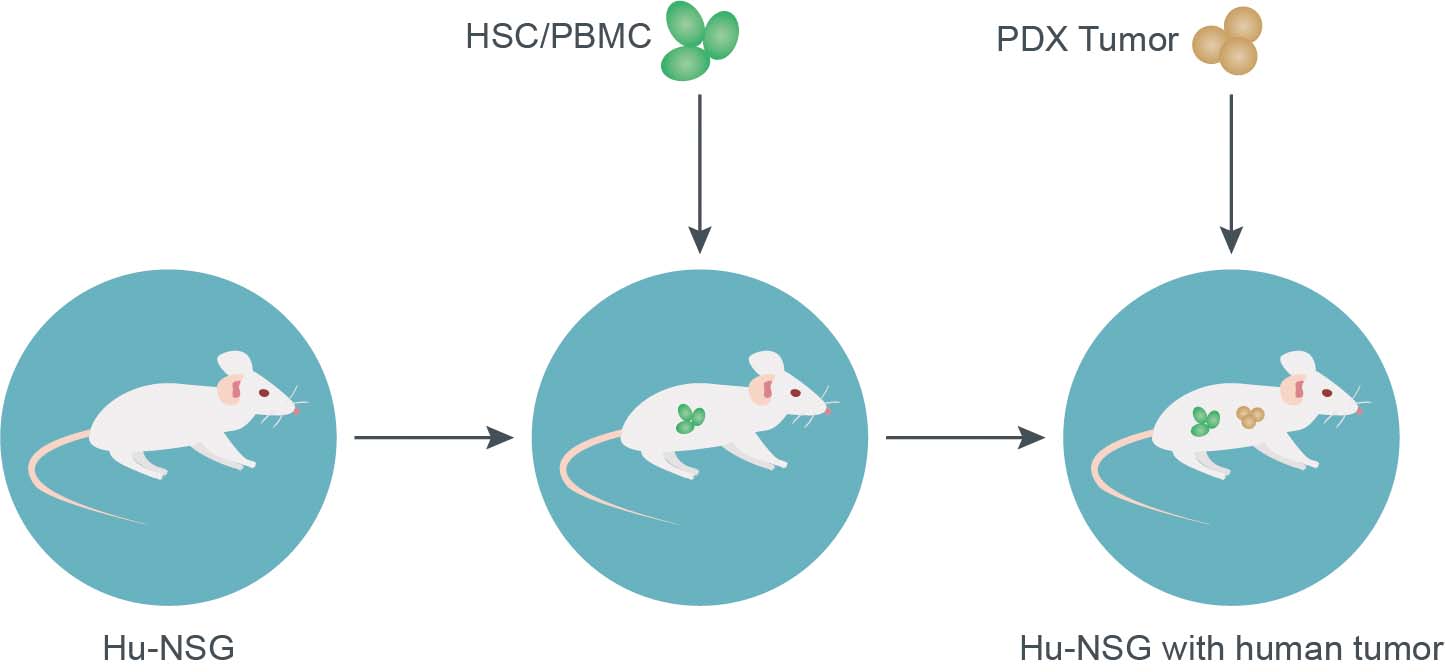
Creative Biolabs has established a preclinical platform for CAR-T cell therapy evaluation which enables us to answer all your preclinical CAR-T questions through our series of translation models. Currently, we provide numerous types of mouse models featuring more complete immune reconstitutions for customers to choose from. Here are some of our mouse models for reference, if you have other needs, please fell free to contact our technical team.
NHP Models for assessing immuno-safety (early toxicity ) of CAR-T therapies
Our NSG mouse models have a higher degree of immunodeficiency. It lacks NK cells while lacking T cells and B cells, and innate immunity is compromised. The NSG model is very suitable for human tumor cell transplantation (CDX), human tumor tissue transplantation (PDX), human peripheral blood mononuclear cell (PBMC), and human hematopoietic stem cell (CD34+HSC) transplantation for immune reconstruction. NCG has a long-life cycle, >89 weeks, which is good for long-term transplantation and pharmacodynamic evaluation.
We use patient-derived xenotransplantation (PDX) models to study population diversity, truly mimic the heterogeneity of human diseases and immunotherapy responses, and enhance the transformative value of CAR-T. PDX is a highly predictable model that can preserve the heterogeneous pathological and genetic characteristics of the original patient's tumor and provide a translatable response. Our collection of more than 2,500 well-characterized PDX models provide a wide range of CAR-T cell therapy targets.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION