We begin with a detailed discussion to understand your goals, target cell line, and desired deliverables. A comprehensive project proposal and timeline are provided
The field of genetic engineering is continuously evolving, moving beyond the limitations of early viral and non-viral delivery systems. While CRISPR-Cas technologies have revolutionized targeted gene editing, the challenge of achieving stable, long-term, and high-efficiency genomic integration of large transgenes remains a significant hurdle in both basic research and therapeutic development. Viral vectors, though efficient, are often constrained by cargo size, immunogenicity, and complex production logistics. Non-viral methods like lipofection or electroporation frequently result in transient expression, limiting their utility for applications requiring sustained genetic modification. At Creative Biolabs, with over two decades of pioneering work in molecular and cell biology, we have perfected the art and science of transposon-based gene delivery.
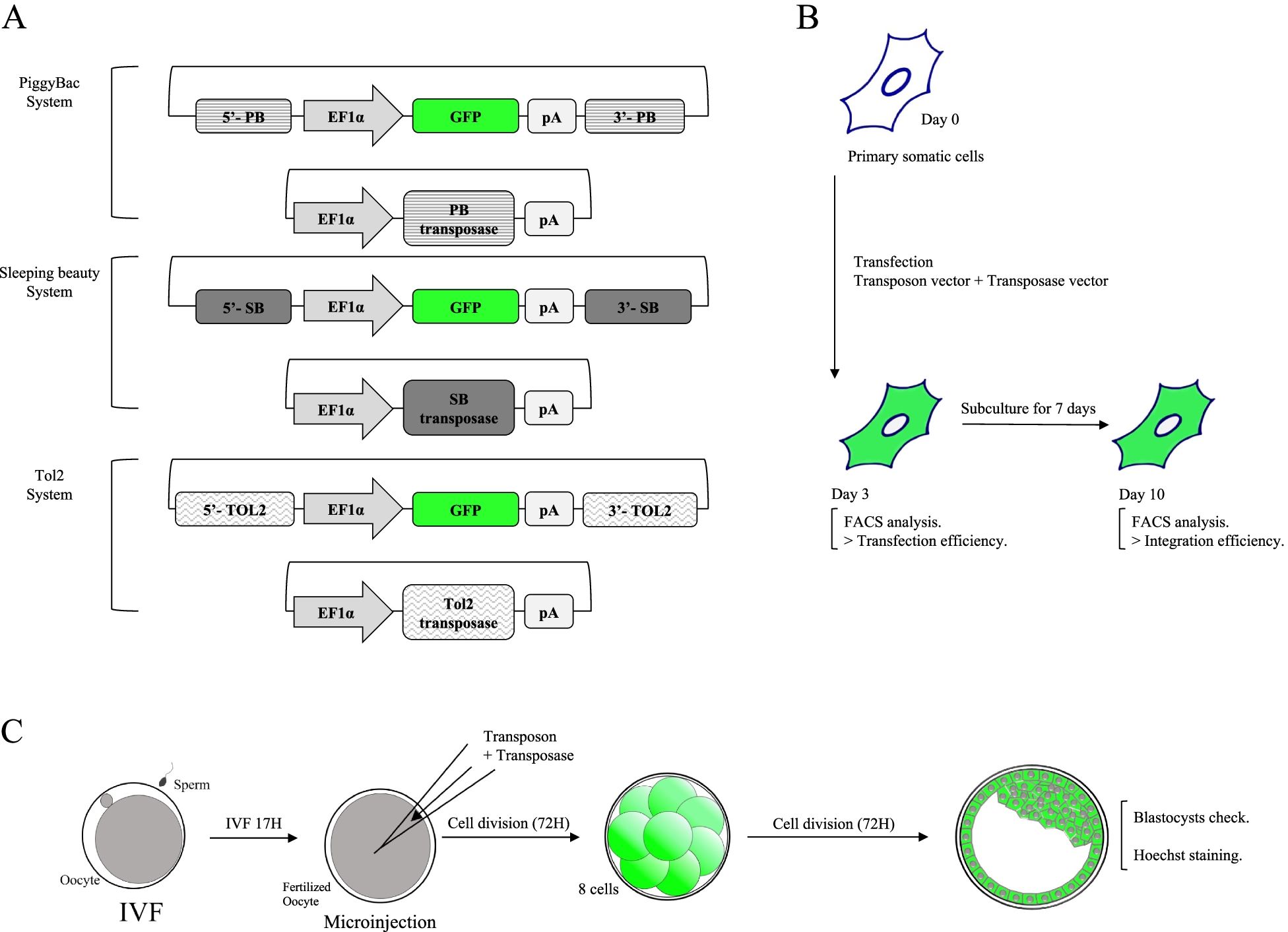 Fig.1 Schematic design of the transposon systems (PB, SB, and Tol2) application in bovine somatic and germ cell1,2
Fig.1 Schematic design of the transposon systems (PB, SB, and Tol2) application in bovine somatic and germ cell1,2
Our suite of Transposons Transfection Services provides a robust, versatile, and scalable platform to overcome these challenges, enabling seamless genomic integration for your most ambitious cellular engineering projects.
We offer an end-to-end solution, tailoring our services to meet the specific needs of each client's project, from initial vector design to final clone validation.
Custom Transposon Vector Construction

Cell Line Transfection & Selection
Single-Cell Clone Isolation & Expansion

Comprehensive Molecular Validation
Choosing our services provides you with a strategic edge, grounded in scientific excellence and operational reliability.
![]() Unparalleled Expertise: Our team of PhD-level scientists brings over 20 years of direct experience in transposon biology, vectorology, and cell line development. We navigate complex project challenges with proven strategies.
Unparalleled Expertise: Our team of PhD-level scientists brings over 20 years of direct experience in transposon biology, vectorology, and cell line development. We navigate complex project challenges with proven strategies.
![]() Superior Integration Efficiency: Our proprietary formulations of hyperactive transposase systems (e.g., SB100X, piggyBac variants) ensure exceptionally high integration rates, leading to a greater yield of stable clones.
Superior Integration Efficiency: Our proprietary formulations of hyperactive transposase systems (e.g., SB100X, piggyBac variants) ensure exceptionally high integration rates, leading to a greater yield of stable clones.
![]() Precision and Footprint-Free Engineering: A key differentiator of our piggyBac-based services is the ability to perform "footprint-free" excision, allowing for clean removal of the transgene, which is invaluable for iPSC reprogramming and reversible genetic studies.
Precision and Footprint-Free Engineering: A key differentiator of our piggyBac-based services is the ability to perform "footprint-free" excision, allowing for clean removal of the transgene, which is invaluable for iPSC reprogramming and reversible genetic studies.
![]() Robust and Scalable Process: From a small-scale research project to GMP-compatible manufacturing for pre-clinical studies, our processes are designed for scalability and reproducibility.
Robust and Scalable Process: From a small-scale research project to GMP-compatible manufacturing for pre-clinical studies, our processes are designed for scalability and reproducibility.
![]() Integrated, End-to-End Support: We are your single point of contact for the entire project lifecycle, from conceptual design to the delivery of validated, banked cell lines, saving you time and streamlining internal resources.
Integrated, End-to-End Support: We are your single point of contact for the entire project lifecycle, from conceptual design to the delivery of validated, banked cell lines, saving you time and streamlining internal resources.
We believe in transparency and clear communication throughout our collaboration.
We begin with a detailed discussion to understand your goals, target cell line, and desired deliverables. A comprehensive project proposal and timeline are provided
Our team designs the optimal transposon construct and proceeds with molecular cloning, followed by rigorous QC (sequencing, restriction digest)
The validated transposon vector and transposase mRNA/plasmid are co-delivered into your target cells. Cells are then transitioned to selection media to establish a stable polyclonal pool
Single-cell clones are isolated and expanded. These clones are screened using high-throughput methodologies to identify top candidates with the desired integration and expression profile
The lead clones undergo in-depth molecular and functional validation. Upon your final review, the validated cell lines and a comprehensive project report are delivered.
Our Transposon Transfection Services are a foundational technology with broad applicability.
|
|
Stable Cell Line Development For high-yield recombinant protein production (e.g., antibodies, vaccines) and long-term functional studies |

|
Gene and Cell Therapy Research Engineering of CAR-T cells, stem cells, and other therapeutic cell types with stable transgene expression for pre-clinical development |

|
Functional Genomics & Screens Creating genome-wide lentiviral-transposon hybrid libraries for high-throughput gain-of-function or loss-of-function genetic screens. |

|
Induced Pluripotent Stem Cell (iPSC) Engineering Safe and efficient integration of reprogramming factors or corrective genes into patient-derived iPSCs. |

|
Disease Modeling Generating isogenic cell lines with specific disease-associated mutations to study pathogenesis and identify novel therapeutics. |
What is the primary advantage of using transposon systems over lentiviral transduction for stable cell line generation?
Transposon systems offer a non-viral alternative, eliminating concerns related to viral vector biosafety, immunogenicity, and the inherent limitations on cargo size. They provide a more straightforward and cost-effective production path, especially for large or complex genetic constructs, while still achieving high-efficiency, long-term genomic integration.
Can your service handle difficult-to-transfect cell types, such as primary cells or neurons?
A: Absolutely. We have developed specialized proprietary protocols and optimized delivery conditions for a wide spectrum of challenging cell types, including primary cells, stem cells, and neurons. Our initial project consultation includes a feasibility assessment to determine the optimal strategy for your specific cell line.
How do you manage the risk of insertional mutagenesis or transgene silencing?
We employ several strategies to mitigate these risks. Our hyperactive transposases promote a more random integration profile, reducing the likelihood of genotoxicity compared to some viral vectors. Furthermore, we utilize donor vectors equipped with chromatin insulators and CpG-methylation minimized backbones to significantly enhance the long-term stability of transgene expression and prevent epigenetic silencing.
What level of characterization and data can I expect upon project completion?
You will receive a final project report that includes detailed documentation of the vector design, full sequencing data, copy number analysis (e.g., via qPCR or Southern blot), and comprehensive expression data (e.g., flow cytometry histograms, Western blot images). We deliver the fully characterized cell clones and their associated banks.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION