Native Ligation Assembly
Native ligation assembly is a practical method that can be widely used in various fields. As a leader in the field of glycoprotein research, Creative Biolabs has mastered a variety of technologies for ligation assembly. We can provide efficient native ligation assembly services to customers around the world.
Native ligation assembly is a practical method that can be widely used in various fields. Recently, considerable efforts have been devoted to exploring the gentle natural chemical linkage (NCL) reactions in regulating peptide native ligation assembly. NCL is an attractive method of protein synthesis, especially suitable for preparing proteins with post-translational modifications. NCL is very similar to the splicing reaction of an intein in the expression of some proteins.
A peptide with a C-terminal thioester reacts with an N-terminal cysteine residue in another peptide to undergo a thioester transfer reaction, which results in the formation of an intermediate thioester with a cysteine thiol. The subsequent nucleophilic attack of the electron-rich nitrogen on the ester carbonyl group results in an S→N shift; then a natural amide bond is formed. A peptide with an N-terminal cysteine side-chain reacts with a peptide with a C-terminal thioester, which results in the formation of an amide bond. This reaction mainly proceeds through the transthioesterification of cysteyl peptides and the subsequent S→N shift, thereby allowing intramolecular amidation.
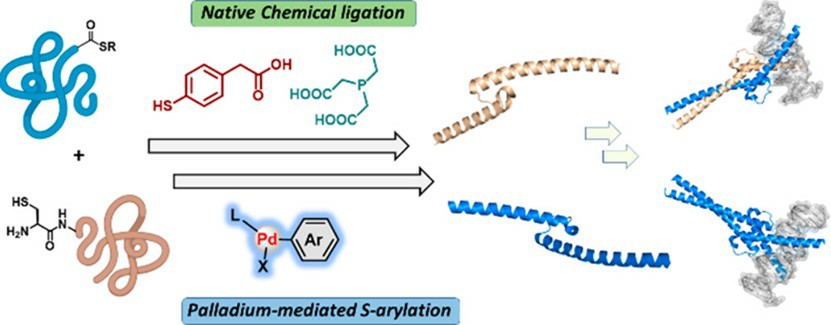 Fig.1 NCL and Pd-mediated peptide ligation strategies.1
Fig.1 NCL and Pd-mediated peptide ligation strategies.1
Advantages of NCL
The NCL reaction can promote the formation of different self-assembled gelling agents through the formation of covalent bonds. Therefore, the application of NCL in peptide self-assembly provides a new idea for the development of soft materials. In addition, NCL plays an important role in the production of complex lipid and phospholipid bilayers. It is also worth mentioning that the NCL reaction has been widely used in macromolecular chemistry to prepare high molecular weight peptide-based biopolymers, multivalent peptide-based dendrimers, hydrogels, or cross-linked micelles.
Applications of NCL
The NCL reaction is closely related to the formation of different self-assembly gelling agents. Therefore, studying the application of NCL in peptide self-assembly provides new ideas for the development of soft materials. In addition, NCL plays an important role in the production of complex lipid and phospholipid bilayers. It is also worth mentioning that the NCL reaction has been widely used in macromolecular chemistry to prepare high molecular weight peptide-based biopolymers, multivalent peptide-based dendrimers, hydrogels, or cross-linked micelles. With the development of the NCL, various thioester compounds can be used in this reaction to form a specifically labeled N-terminal cysteine peptide.
Services in Creative Biolabs
In summary, NCL provides a promising and efficient method for chemically selectively linking unprotected peptides and protein fragments to produce proteins. As experts in this field, our scientists are very professional in the basic knowledge of NCL and pioneering methods in chemical protein synthesis. We can provide various services to meet the requirements of different projects. Our services range from the development of thiolated and selenized amino acids to the post-translational modification of peptides and proteins incorporated through NCL. Please contact us for more details.
Reference
-
Lin, Xiaoxi, et al. "Enabling Peptide Ligation at Aromatic Junction Mimics via Native Chemical Ligation and Palladium-Mediated S-Arylation." Organic Letters 25.25 (2023): 4715-4719. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 NCL and Pd-mediated peptide ligation strategies.1
Fig.1 NCL and Pd-mediated peptide ligation strategies.1

