Glycoprotein Synthesis
Biological Importance of Glycoproteins
The elaborate carbohydrate constructs observed on the surface of cells and proteins hold the key to understanding many complex biological processes. For example, glycoproteins have been reported to exhibit a pivotal role in processes as diverse as fertilization, neuronal development, hormone activities, immune surveillance, and inflammatory responses. In addition to their critical role in communication events, an interesting aspect of N-linked glycans is highlighted by their post-translational role in the “quality control” of protein synthesis. In the absence of correct glycosylation, many proteins fold incorrectly. Carbohydrates can also alter other physiochemical properties of proteins. Alterations of amino acid side chains such as glycosylation lead to higher structural and functional protein diversity and are therefore a prime candidate in explaining the seeming incongruity in gene number and biological function.
Chemical and Biological Approaches to Glycoprotein Synthesis
The chemical synthesis of glycopeptides has become possible in recent years as milder deprotection methods have been developed for glycopeptide synthesis. Thus, it is now possible to incorporate glycosylated amino acids during solid-phase synthesis. This is particularly useful for small oligosaccharide sidechains, such as those found in O-linked glycans. Alternatively, suitably protected peptides can be synthesized first, then chemically glycosylated. This is particularly useful for N-linked oligosaccharides.
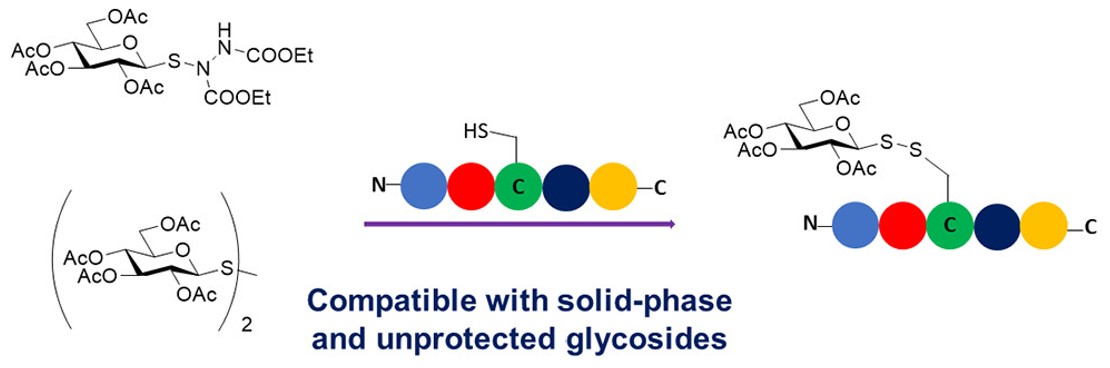 Fig.1 Solid-phase synthesis of glycopeptides.1, 4
Fig.1 Solid-phase synthesis of glycopeptides.1, 4
Glycosidases and glycosyltransferases are not only integral to in vivo glycosylation, but also useful for in vitro processing. A range of these enzymes has been made available through isolation from natural sources or by heterologous overexpression in foreign hosts. These enzymes have been shown to accept saccharides, glycolipids, and glycoproteins as substrates. By using an excess of the enzyme, high yielding reactions are possible, even with poor substrates.
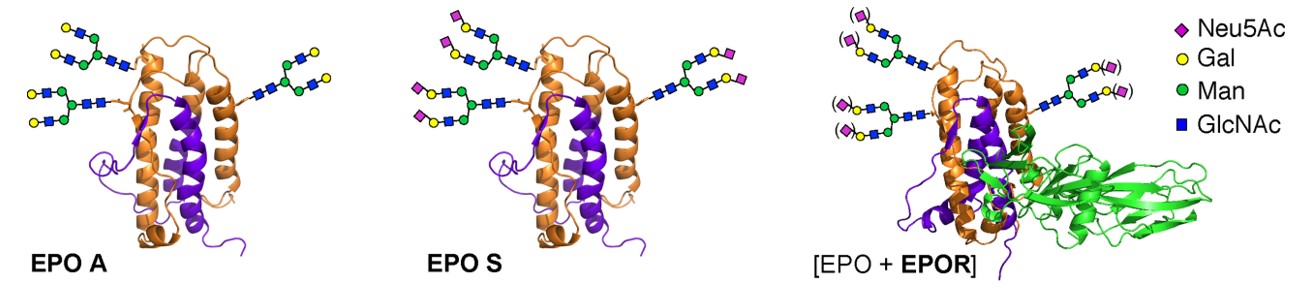 Fig.2 Two glycoforms of human erythropoietin (EPO) synthesized by chemoenzymatic method.2, 4
Fig.2 Two glycoforms of human erythropoietin (EPO) synthesized by chemoenzymatic method.2, 4
The biosynthesis of glycoproteins is well documented and is similar in all eukaryotes. Each organism has its own “glycosylation machinery” and thus expression of the same gene in different organisms produces different glycoproteins. Moreover, the efficiency of one or more biosynthetic steps may be low, resulting in a mixture of oligosaccharide side chains of different lengths and compositions at each glycosylation site. Therefore a glycoprotein is generally a heterogeneous mixture of “glycoforms”, protein molecules that differ only in the structure of the bound oligosaccharide.
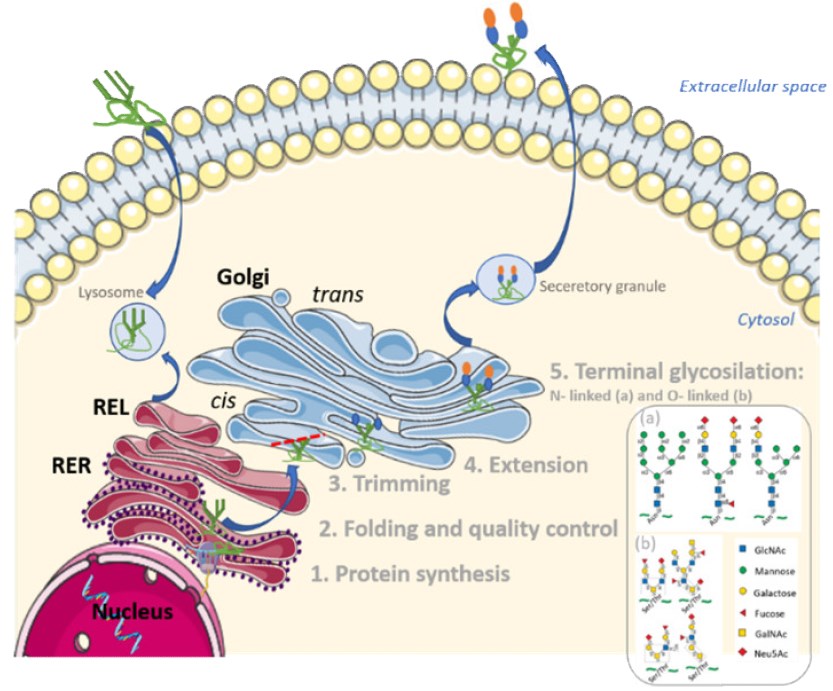 Fig.3 Initiation and maturation of glycoproteins.3, 4
Fig.3 Initiation and maturation of glycoproteins.3, 4
There is a remarkable diversity of techniques used to construct glycoproteins. The complexity of synthesis remains an issue, but with wisdom and a combination of some complementary techniques, practical synthesis of complex glycoproteins will be achieved. Since glycosylation has an important role in correct protein folding, it is unlikely to be enough to “just make it”, and in this regard, the timing of glycosylation and assembly strategy in any synthesis may too become a vital issue. In addition to the preparation of naturally occurring glycoprotein structures, we may now also start to use glycosylation on sites that are not normally glycosylated as tools for creating novel protein functions and architecture.
Creative Biolabs has been dedicated to glycoprotein research for many years. We are flexible in serving customers' glycoprotein synthesis projects, providing a variety of reasonable solutions for customers to make the best choice. Our team of senior scientists and experienced technical team have helped many customers overcome the multiple challenges of glycoprotein synthesis. For more details, please feel free to contact us or send us an inquiry directly.
References
-
Banisalman, Katreen AF, et al. "Chemoselective Solution-and Solid-Phase Synthesis of Disulfide-Linked Glycopeptides." The Journal of Organic Chemistry 87.21 (2022): 14026-14036.
-
Hessefort, Hendrik, et al. "Chemical and enzymatic synthesis of sialylated glycoforms of human erythropoietin." Angewandte Chemie International Edition 60.49 (2021): 25922-25932.
-
Fuertes-Martín, Rocío, et al. "Human serum/plasma glycoprotein analysis by 1H-NMR, an emerging method of inflammatory assessment." Journal of clinical medicine 9.2 (2020): 354.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 Solid-phase synthesis of glycopeptides.1, 4
Fig.1 Solid-phase synthesis of glycopeptides.1, 4
 Fig.2 Two glycoforms of human erythropoietin (EPO) synthesized by chemoenzymatic method.2, 4
Fig.2 Two glycoforms of human erythropoietin (EPO) synthesized by chemoenzymatic method.2, 4
 Fig.3 Initiation and maturation of glycoproteins.3, 4
Fig.3 Initiation and maturation of glycoproteins.3, 4

