Synthesis of N-glycopeptides
The Introduction and Necessity for Glycopeptide Synthesis
Glycopeptides refer to the peptides that contain glycans covalently attached to the side chains of the amino acid residues that constitute the peptide. In the past decades, glycans on the cell surface and glycoproteins play a critical role in biological research, including the immune system, brain development, the endocrine system, fertilization, as well as inflammation. Therefore, the synthesis of glycopeptides provides biological probes for researchers to clarify the functions of glycans in nature and develop products with biotechnological applications.
The Classical Technologies for N-glycopeptides Synthesis
N-glycosylation is an important protein posttranslational modification type to affect a series of biological functions and immunity. During this process, the oligosaccharide would be transferred to asparagine within the consensus sequence Asn-X-Ser/Thr. It has been proved that the natural N-glycans in glycopeptides can protect the peptides from proteolysis. To better understand the biological functions of N-glycosylation, efficient approaches for N-glycopeptides synthesis are necessary.
To achieve this goal, there is the linear, convergent, and thioacid-mediated synthesis of N-glycopeptides. In the classical linear method, a pre-glycosylated aspartic acid derivative would be applied in solid-phase peptide synthesis (SPPS). The large glycopeptides can be obtained via the convergent approach while the peptide is glycosylated after SPPS. N-linked glycopeptides can be synthesized by the ammonolysis polymerization of ω-Asp p-nitrophenyl thioester in solution. In the thioacid-mediated approach, the side-chain carboxyl group of aspartic can be replaced using a thiocarboxylic acid (thioacid) to obtain N-glycopeptides selectively and also avoid further protecting-group manipulations.
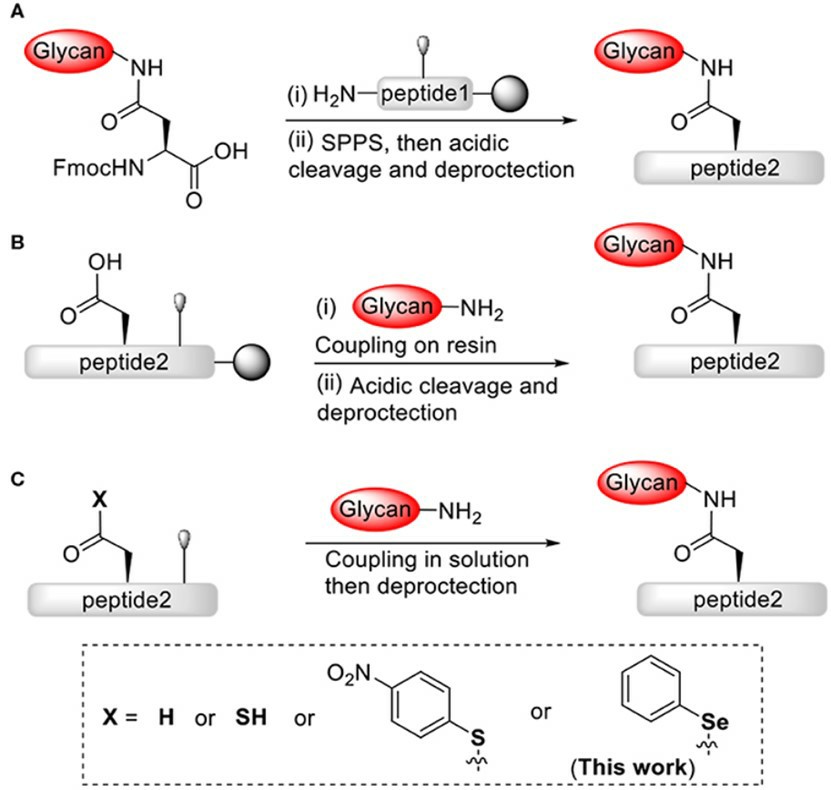 Fig.1 An efficient strategy for chemical construction of N-linked glycopeptides.1
Fig.1 An efficient strategy for chemical construction of N-linked glycopeptides.1
The Novel Tripeptide Building Block Approach for N-glycopeptides Synthesis
The novel-developed tripeptide building block approach allows the synthesis of aspartic thioacid-containing peptides via fluorenylmethyloxycarbonyl (Fmoc)-SPPS. According to a chemoselective thioacid-glycosylamine ligation, these peptides can be converted to N-glycopeptides successfully. The high chemical selectivity of N-glycopeptide provides an alternative to the tedious manipulation of protecting groups for full-length peptides. In addition, the discovered novel thioacid-mediated peptide bond cleavage reaction provides interesting new insights into the chemistry and applications of peptide thioacids, thereby bringing new perspectives to peptide chemistry.
Creative Biolabs has been a long-term expert in the field of glycomics. As a pioneer and the undisrupted global leader in glycan research, we offer a variety of products and services including custom glycoprotein synthesis. If you are interested in our products or services, please do not hesitate to contact us for more detailed information.
Reference
-
Du, Jing-Jing, et al. "Peptidyl ω-Asp selenoesters enable efficient synthesis of N-linked glycopeptides." Frontiers in Chemistry 8 (2020): 396. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Resources

 Fig.1 An efficient strategy for chemical construction of N-linked glycopeptides.1
Fig.1 An efficient strategy for chemical construction of N-linked glycopeptides.1

