Induced pluripotent stem cells (iPSCs) are a series of adult cells that have been commonly reprogrammed to present embryonic-like stem cells (ESCs) properties. Pilot studies have indicated that iPSCs can be considered as an unlimited source of various human cells in disease treatment. Human iPSCs overcome the ethical issues caused by ESCs and are easily accessible to the large production of patient-derived somatic cells.
As a result, the iPSC technology has been broadly used for a wide range of therapeutic purposes, such as drug discovery, safety assessment, tissue engineering, disease modeling, as well as stem cell therapy. Besides, a wide array of analyses has demonstrated that the differences between ESC and iPSC can reflect on human allelic diversity. The data have suggested that the diversity of iPSCs can seriously affect their applications in autologous therapy and hematopoietic stem cell transplant. In recent years, a panel of iPSC characterization assays has been generated for evaluating the biological properties, heterogeneity, differentiation potential, iPSC genome integrity, and pluripotency in the process of in vitro iPSCs culture.
IPSC technology is an inspiring new idea that plays a significant role in the generation of stem cell therapies against many human diseases. Till now, many efforts have been made to develop novel reprogramming methods and producing clinical-grade iPSCs from different human cell types. Therefore, the combination of integrated IPSC development workflows and comprehensive iPSC characterization assays is necessary for quality assurance in clinical use. In particular, the common methods for completely characterize resulting iPSCs, including not limited to:
Recent studies have suggested that the development of effective stem cell therapies for treating human diseases requires fully understand the human genetic variation during the disease progress. Pluripotent stem cells can be regarded as an attractive tool for providing disease-associated cell types to model specific diseases.
In general, the methods for iPSC pluripotency analysis are time-inefficiency, high cost, and usually require reprogramming. Moreover, a large amount of patient-specific pluripotent samples is needed to test the relationship between genetic mutations and disease development. Nowadays, with the development of large iPSC banks, a wide variety of cost-effective, high-throughput technologies, such as HLA typing, karyotype, single tandem repeat (STR) genotyping, and whole-genome sequencing (WGS), to characterize iPSC pluripotency phenotype has become a major trend.
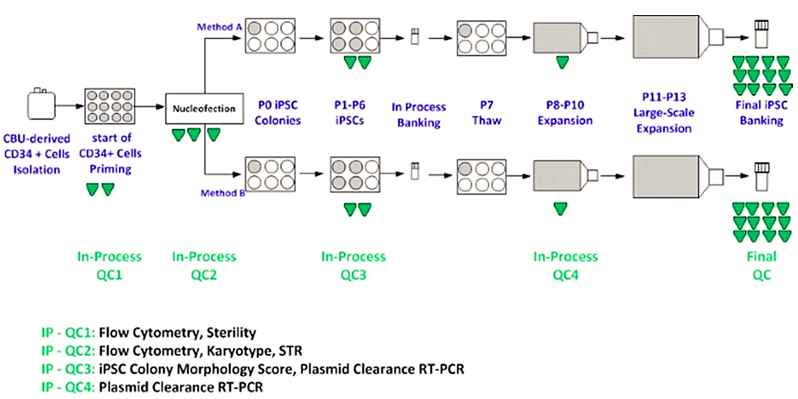 Fig.1 Human iPSC manufacturing process flow diagram with in-process testing of samples.1
Fig.1 Human iPSC manufacturing process flow diagram with in-process testing of samples.1
Techniques are now accessible to characterize iPSCs and test their genetic integrity in vitro under defined culture conditions without change of phenotype or loss of function. Below are the main assays for iPSC characterization.
Reference
For Research Use Only. Not For Clinical Use.