Cancer vaccines typically are complex mixtures of antigens, epitopes, adducts, and impurities, making the reproducibility of the vaccine substance dependent on the process of production. The final vaccine manufacturing process is determined by process development, equipment selection, and facility design.
The cancer vaccine production and product licensing depend on the process, equipment, and facilities. In Creative Biolabs, this process development for vaccine production involves pharmaceutic research, process evaluation, manufacture optimization, stability tests, assay validation, product packaging and other practical activities required to convert an antigen into a vaccine. We are operating in accordance with international practice, which defines the vaccine and contributes to creating a new robust, reproducible cancer vaccine manufacturing process for long-term vaccine supply.
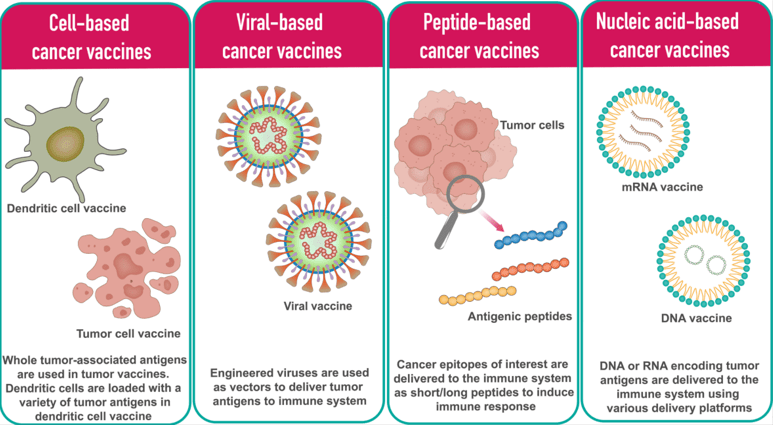 Various cancer vaccine platforms.1
Various cancer vaccine platforms.1
The cancer vaccine products are diverse and complex, including live attenuated vaccines, inactivated vaccines, subunit vaccines, therapeutic vaccines and so on. There are three major stages in the vaccine development, studies leading to antigen discovery, development leading to small-scale manufacturing and manufacturing leading to large-scale production of vaccines. With the help of us, well-designed processes and dedicated facilities can be used to produce very large quantities of low-cost, high-quality cancer vaccines for a broad range of needs.
The development of an expression system with high efficiency, scalability, and repeatability is important for the manufacture of cancer vaccines. Notably, Creative Biolabs hopes to introduce general information on protein expression for cancer vaccine development and assists clients to access the optimal expression system for specific applications.
Classical systems:
Alternative systems:
The vaccine industry benefits from the advancement of purification technologies, such as membrane technology, disposable technology, process automation, high-throughput process development, process data tracking, analysis, etc. With significant evolution and breakthrough in bioprocess technologies, Creative Biolabs has improved traditional methods for cancer vaccine production process in the last few years and proposed many innovative solutions for downstream vaccine purification.
The cancer vaccine process development starts at a laboratory scale for the identification of unit operations and parameters, then gradually expands the scale (usually 20L fermentation) to produce GMP materials for Phase I clinical studies. Process definition studies are performed on the key unit operations and parameters using the experimental design before scaling up to 200 L (normally for Phase II). Prior to Phase III (about 2000L scale), the process validation and engineering must be carried out. In Creative Biolabs, we will perform biophysical and chemical characterization to demonstrate product comparability and process scalability during the scale-up and process development.
To understand and control cancer vaccine production process better, along with predict performance of mass production.
To optimize cancer vaccine production processes as requests and obtain mass production of final vaccine products with low costs.
For many years, Creative Biolabs has consistently devoted to the global vaccine industry with a wide range of superior products and services. We can provide a diversity of cancer vaccine production process development services to ensure a long-term and large-scale supply of high-quality vaccines for worldwide uses.
If there is no relevant service you're interested in, please contact us for more information.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION