An adjuvant (from the 'adjuvare' of Latin, meaning 'help' or 'enhancement') is defined as any compound that is added to the vaccine to enhance immune responses against antigens. It is an essential component of many vaccines and is typically used to enhance immunity against antigens, which is impressively important for populations with reduced immune responses, including those with hypo-responsiveness to vaccination, and for those who are naive to the pathogens. In addition, this ability can serve to increase the viability of potentially promising vaccine approaches (e.g. recombinant protein vaccines) and to allow antigen dose-sparing, which helps to streamline production by overcoming limitations in manufacturing processes.
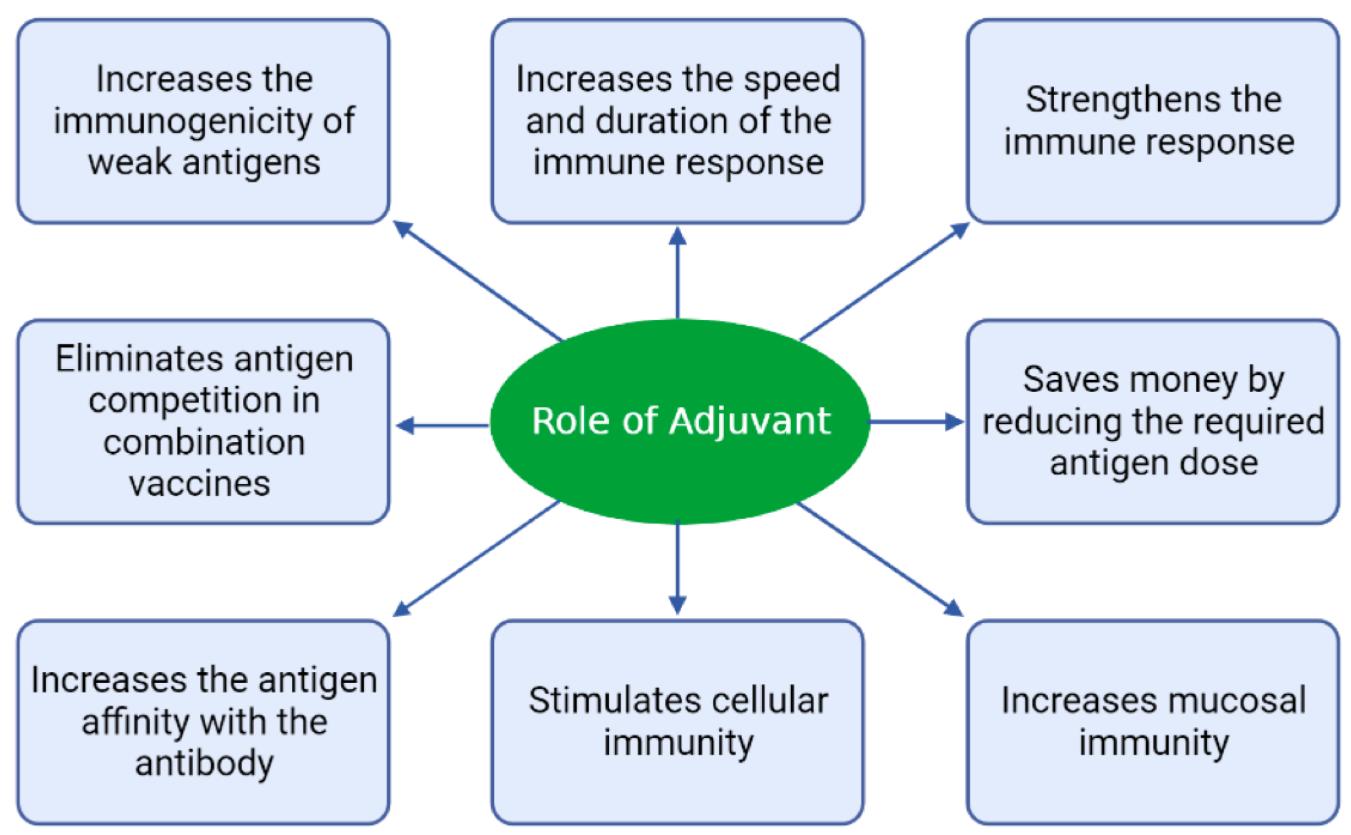 Fig.1 Characteristic properties of vaccine adjuvants.1
Fig.1 Characteristic properties of vaccine adjuvants.1
Cancer Vaccine adjuvants market is segmented into pathogens, particulates, emulsion adjuvants, combinations, and others. In Creative Biolabs, we continuously make efforts to unravel the modes of action of different adjuvants, in order to access opportunities for maximizing the potential of new cancer vaccines. Meanwhile, new adjuvants and adjuvant formulations are being developed by us as our understanding of immunogenicity and complementary mechanisms continue to deepen.
The vaccine adjuvant is any product or combination of components that can increase or modulate the humoral or cellular immunity. In many cases, the immunogenicity of antigens is very weak, thereby an adjuvant is required to increase the immune response. Creative Biolabs has strong capability to develop potent adjuvants that improve the effectiveness of cancer vaccines and reduce the cost of vaccination programs. Here, we hope to introduce a number of robust services for global scientists during adjuvant development to accelerate their projects in specific vaccine studies.
And there are various kinds of cancer vaccine adjuvants that can be provided by us, including but not limited to:
| Mineral salts | Aluminum salts (alum), calcium phosphate; |
| Lipid particles | Complete Freund's adjuvant (CFA); incomplete Freund's adjuvant (IFA); MF59; AS03; cochleates |
| Microparticles | Virus-like particles (VLP); virosomes; PLA (polylactic acid); PLG (poly[lactide-coglycolide]) |
| Immune potentiators | dsRNA; monophosphoryl lipid A (MPL); LPS; flagellin; imidazoquinolines; CpG oligodeoxynucleotides (ODN); muramyl dipeptide (MDP); saponins (QS-21) |
| Mucosal adjuvants | Cholera toxin (CT); heat-labile enterotoxin (LTK3 and LTR72); chitosan |
Adjuvants have several key benefits, such as reducing the number of antigens required, cutting the number of vaccines needed, enhancing the vaccine effectiveness in immunocompromised people, and so on. Creative Biolabs aims to promote classical and novel cancer vaccine adjuvants toward licensing for human uses, and also support the optimization of adjuvant candidates and research of pharmacology, toxicity, and efficacy of pre-clinical adjuvant.
Please feel free to contact us for more information on adjuvant development services.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION