The tumor microenvironment (TME) plays a critical role in the progress of tumors. TME undergoes various alterations, which result in a change in pH, oxygen level, and chemokine gradients. These changes result in dysplasia, which is the appearance of a heterogeneous population of tumoral cells with different genetic and phenotypic traits. It has been reported that the hyporesponsiveness or unresponsiveness of patients to tumor immunotherapy may be due to the heterogeneity of the tumor immune microenvironment. Remodeling the tumor immune microenvironment is an important strategy to lift the immunosuppression and achieve immune normalization. In addition, has a recognized capability to hamper the penetration of conventional drugs and drug delivery systems because of the tumor growth process itself.
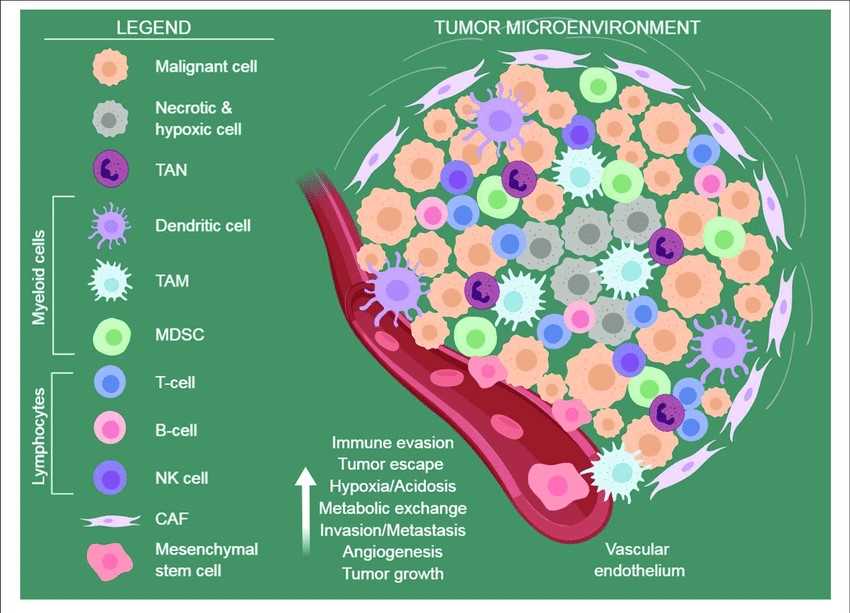 Fig.1 The TME.1,3
Fig.1 The TME.1,3
The advancements in the understanding of the TME have also led, in recent years, to the development of efficacious therapies to treat cancer. In contrast to cancer cells, stromal components in the TME are generally stable in genetics and represent a potentially ideal target for therapeutic intervention. Currently, cancer treatment strategies targeting the TME are mainly focused on different molecular targets, special cells or intercellular ingredients in the TME. Among them, cytokines, growth factors and survival-associated proteins released by the TME are straightforward and valid therapeutic targets. The related approaches of chemotherapy, targeted therapy, immunotherapy, gene therapy, traditional Chinese medicine, combined drug therapy, and nanotechnology have been applied to key molecular targets in the TME, thus inhibiting the promoting effect of the TME on cancer occurrence, development and metastasis. However, the TME remodeling effects of currently available therapies remain largely unexplored in many previous reports.
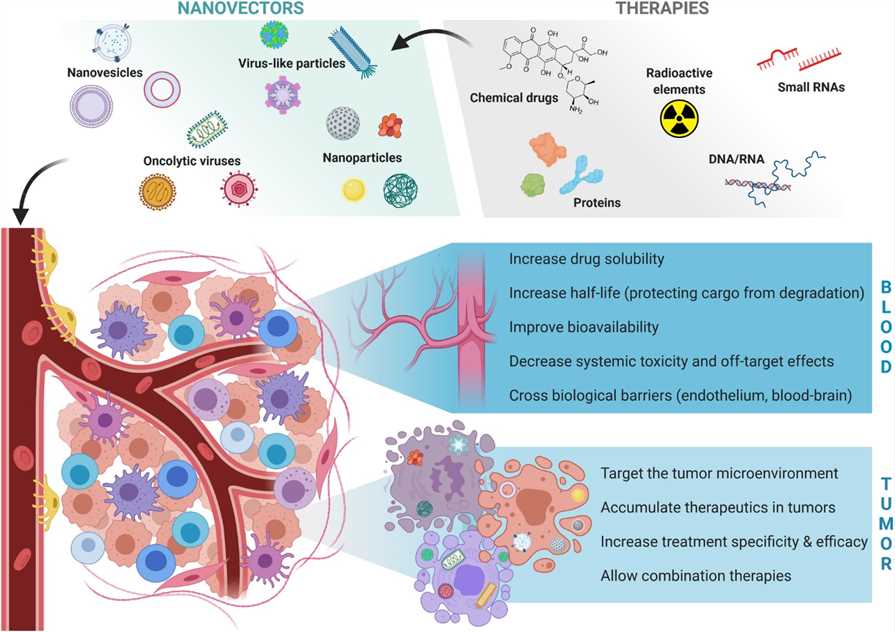 Fig.2 Advantages of vectorization for delivering cancer therapies.2,3
Fig.2 Advantages of vectorization for delivering cancer therapies.2,3
Many small-molecule inhibitors have been developed to specifically or mainly target TME. These small molecules are designed to target components of the TME and interrupt the specific features of TME, including the hypoxic, acidic, inflammatory milieu, as well as the abnormal ECM network in TME.
Antibodies are extremely versatile as platforms for the development of novel therapeutics which has resulted in a large diversity of approaches. Recombinant technology offers enormous opportunities to tailor antibodies to meet clinical requirements. Targeting the TME with antibodies represents a compelling strategy to inhibit pro-tumorigenic processes.
Nucleic acid therapeutics are a diverse class of DNA or RNA such as plasmids, mRNA, siRNA, miRNA, small-activating RNA (saRNA), gene-editing gRNA, etc. The nucleic acids as a therapeutic mean can exert functions by reprogramming constituents of the tumor microenvironment by gene transfer, gene editing and RNA interference (RNAi).
With the advantages of controlled delivery, enhanced permeability and retention (EPR) effect and modular flexibility, nanomedicine has offered opportunities to strengthen antitumor immune responses and to sensitize the tumor to immunotherapy. Nanomedicine can be designed to target tumor cells or immune cells and respond to a broad palette of environmental and physiological stimuli in cellular vicinities (pH, reactive oxygen species (ROS), Hypoxia, enzymes, etc). In addition, nanomaterials can act as the delivery system to deliver anticancer molecules.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION