The tumor microenvironment (TME) contains many factors that are known to inhibit anti-tumor immune responses, promote tumor cell growth, and induce pro-tumorigenic angiogenesis. Targeting these critical pro-tumorigenic processes within the TME has proved clinically efficacious. Most of the antibody drugs are monoclonal antibodies, as well as antibody-drug conjugates, bispecific antibodies. The clinically most successful antibodies exert antitumor activity either by targeting tumor cells directly (direct-targeting antibodies) or by targeting and activating immune cells that seek up and kill cancer cells in the TME (immune checkpoint antibodies).
The TME participates in all stages of tumor progression and has emerged as a promising therapeutic target for cancer therapy. Novel targets within the TME have been uncovered that can help direct and improve the actions of various cancer therapies. Among them, an antibody recognizing a component of the TME, the extra domain B (EDB) of fibronectin, was put on the top-ten list of "Antibodies in 2018 to watch" as therapeutics for late-stage cancer.
Therapeutic antibodies and immune checkpoint inhibitors offer the best hope of maximizing the clinical benefit by targeting the immunosuppressive TME. Most immune checkpoint inhibitors currently used in the clinic or clinical trials are antibody drugs, for instance, antibodies binding to programmed death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). In addition, combination therapy targeting both pathways simultaneously has been suggested as potentially more efficacious than either therapy alone.
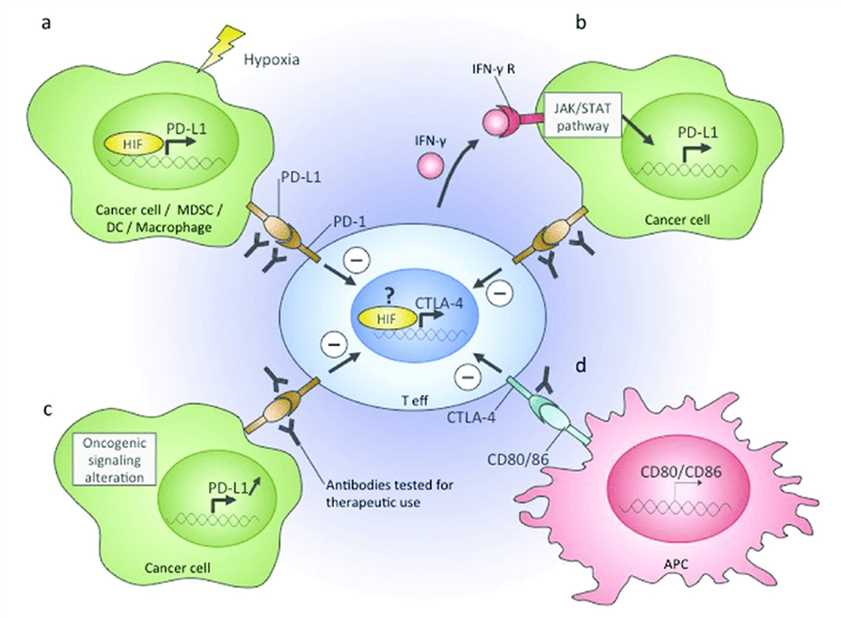 Fig.1 Immune checkpoints in the TME. (Petrova, 2018)
Fig.1 Immune checkpoints in the TME. (Petrova, 2018)
Vascular abnormalities in TME can suppress antigen presentation and immune effector cells, or augment the immune suppressive activity. In turn, immunosuppressive cells can drive angiogenesis. Thereby antiangiogenic therapy can promote antitumor immunity. Many antiangiogenic drugs directly have been tested in clinical trials. The antibody that targets vascular endothelial growth factor (VEGF), was the first anti-angiogenic approved by FDA that is already in the clinics. The targeting of VEGF signaling has been observed to induce tumor vasculature normalization, which in turn can increase extravasation and the number of tumor-infiltrating lymphocytes (TILs) following adoptive cell transfer and cancer cell vaccine. Vascular normalization may also help to prevent the differentiation of TAMs toward an immune inhibitory M2-like phenotype and could block VEGF-mediated inhibition of DC maturation.
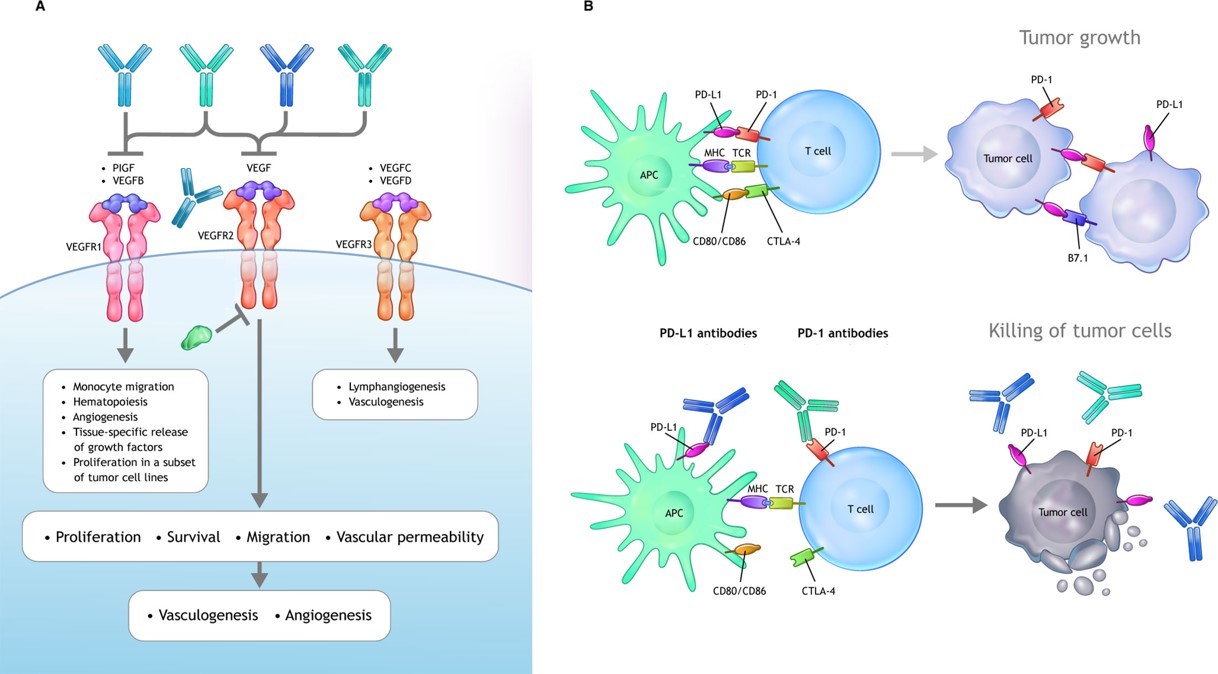 Fig.2 VEGF signaling axes and antibody drugs. (Hack, 2020)
Fig.2 VEGF signaling axes and antibody drugs. (Hack, 2020)
Creative Biolabs has extensive expertise in the field of antibody engineering, production, and bio-conjugation. We are dedicated to serving the unique needs of our clients in therapeutic antibody development projects. Our scientists are confident in accelerating every step of your antibody drug development.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION