Neutrophils, the biggest number of all kinds of white blood cells, take part in defense against infection in innate adaptive immunity. It is generally recognized that neutrophils are attracted to the site of inflammation by chemotactic substances when inflammation occurs. Upon arrival at the site of the inflammatory response, the lamellae of neutrophils contact infectious agents producing chemokines, form pseudopods around them, and form phagosomes phagocytizing foreign bodies. The IgG Fc receptor and complement C3 receptor on the surface of the cell membrane accelerate phagocytosis of the invading foreign body. Furthermore, neutrophils respond to parasite and foreign antigen invasion through IFN-γ/TNF mediated post-oxidative cytotoxic effector molecules and cell particle contents release. Due to their considerable defense capability, neutrophils are considered to be the first line of cellular defense in the body against the invasion of pathogenic microorganisms and play an important role in the occurrence and development of inflammation. Actually, like a professional killer, neutrophils do not participate in antigen presentation but can release cytokines to dissolve connective tissue, which is fatal to invasive pathogens.
Dialectically, the activation of neutrophils is a double-edged sword. Pro-inflammatory and autoimmunity-enhancing factors could be activated by the over-action of neutrophils, among them systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and ANCA-associated vasculitis (AAV). A review has been published in recent years and concluded that there are two mechanisms proposed to explain the role of neutrophils in the pathogenesis of autoimmune disease. 1) Neutrophil extracellular traps (NETs), fibrous networks assembled by nuclear and granular components, can induce "NETosis," which is characterized by special forms of cell death due to active neutrophils; 2) Metabolite pathways result from NET degradation caused by DNase I. However, it is because of the powerful inflammatory-inducing capacity of neutrophils that their immune function is explored in the treatment of tumor-related diseases. In recent years, more and more research has focused on the application of neutrophils in tumor immunotherapy, which is considered a breakthrough concept for cancer therapy. It was reported that training the innate immune system with β-glucan could produce innate immune cells, specifically neutrophils, that could be used in animal models to prevent and attack tumors. Mechanically, the anti-tumor effect of β-glucan induced trained immunity is associated with transcriptomic and epigenetic rewiring of granulopoiesis and neutrophil reprogramming toward an anti-tumor phenotype. This process requires type I interferon signaling, irrespective of adaptive immunity in the host. Adoptive transfer of neutrophils from β-glucan-trained mice to naive recipients suppressed tumor growth in the latter in a ROS-dependent manner.
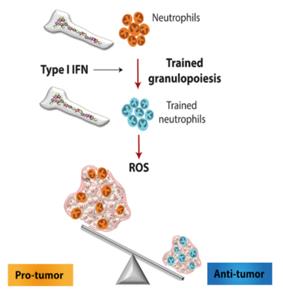 Figure 1. Neutrophils are involved in immunoregulation to inhibit tumor development.
Figure 1. Neutrophils are involved in immunoregulation to inhibit tumor development.
Neutrophils play anti-cancer roles via different mechanisms: 1) the induction of antibody-dependent cellular cytotoxicity (ADCC), 2) direct cytotoxic effect and 3) the activation of antitumor adaptive immunity.
The use of monoclonal antibodies (mAbs) has become a mainstream strategy for the treatment of cancer in immunotherapy. Fc receptors (FcR) on innate immune cells (including neutrophils, macrophages, and natural liller cells) are activated by the binding of monoclonal antibodies (mAbs) and then elicit tumor elimination via the ADCC process.
Neutrophils are capable of expressing Fc receptors for both IgG (FcγR) and IgA (FcαR), leading to the release of tumoricidal mediators through FcR-mAb binding interactions with mAb-opsonized target cells. Recent research suggests that the interaction between neutrophil FcαR1 with IgA mAbs or FcαR1 BsAbs can promote the recruitment of neutrophils into tumors by regulating the release of leukotriene B4 (LTB4). Upon neutrophil stimulation, pro-inflammatory cytokines, such as interleukin 1beta (IL-1β) and TNF-α, are released. Additionally, endothelial cells in tumor colonies were found to produce CXCL8, a chemokine produced by tumor cells, which further facilitates neutrophil migration and enhances the tumor-killing effects.
Neutrophils can inhibit tumor growth through direct cytostatic and/or cytotoxic effects. In 2018, it was reported that neutrophils suppress the proliferation of A549 cells via the Fas/Fas ligand pathway-mediated cell cycle arrest. Additionally, oxidative damage caused by reactive oxygen species (ROS) from activated neutrophils also contributes to tumor eradication. The ROS include single oxygen (1O2), superoxide anion (O2·−), hydrogen peroxide (H2O2), hydroxyl radical (HO−) and nitric oxide (NO), which play an essential role in neutrophil-mediated tumor lysis. Furthermore, β-glucan-induced training of granulopoiesis, as mentioned earlier, could exert an anti-tumor effect in a ROS-dependent manner, given the crucial function of NETs in it. Another study showed that NETs could capture tumor cells and repress their metastatic and proliferative abilities in melanoma cells. Moreover, in neutrophil-deficient colon tumors, researchers found that neutrophils could slow colon tumor growth and progression by restricting the numbers of bacteria and tumor-associated inflammatory responses.
Upon recruitment into inflamed tissue, neutrophils engage in complex interactions with various immune cells, including natural killer (NK) cells, dendritic cells (DCs), B and T lymphocytes. For example, when stimulated by Toll-like receptors (TLRs), neutrophils can promote the maturation of DCs, which in turn leads to the proliferation of T cells and the release of IFN-γ. Additionally, factors such as granulocyte-macrophage colony-stimulating factor (GM-CSF) and IFN-γ can drive the differentiation of immature CD11b+CD15hiCD10−CD16int/low neutrophils into antigen-presenting cell (APC)-like hybrid neutrophils in early-stage human lung cancer. These APC-like neutrophils express CD86 and HLA-DR, which enhance the anti-tumor effect of T cells. The MHC class I receptor on the APC-like neutrophils can also interact with TCR on CD8+ T cells. The resulting activated population of immune cells can then secrete cytokines, including IFN-γ and IL-12, which work together to mount an effective anti-tumor response.
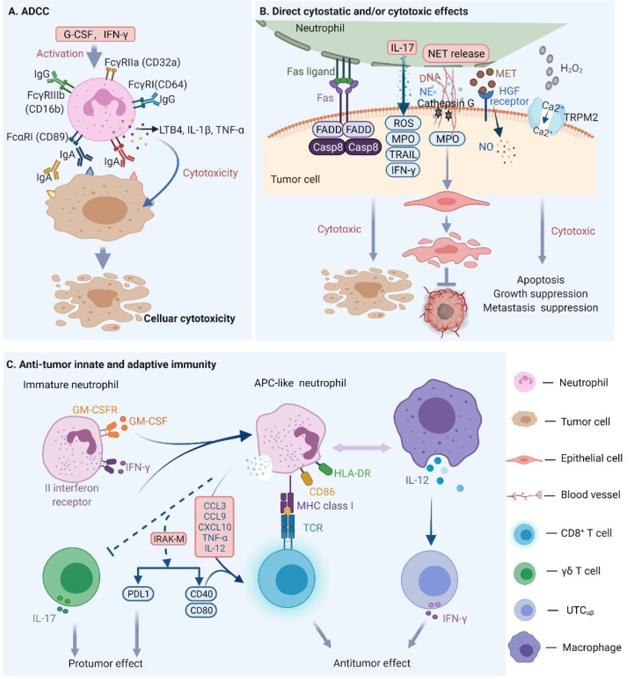 Figure 2. Mechanisms of neutrophil mediated antitumor effects.
Figure 2. Mechanisms of neutrophil mediated antitumor effects.
Understanding the mechanisms behind neutrophil-targeted tumor therapy holds the key to developing effective immunotherapies. Currently, it is clear that PD-1, a star molecule, plays a crucial role in neutrophil-mediated immunotherapy. One approach is to block the immune checkpoint in the PD-1/PD-L1 (programmed cell death 1) pathway of neutrophils. In addition to impacting adaptive immune cells, it has been observed that PD-L1+ TANs (tumor-associated neutrophils) directly interact with PD1+ NK cells and dampen their anti-tumor immune activity in murine colon cancer models. Neutrophil-associated cytokines, such as G-CSF, VEGF, and TGF-β, are classic targets for cancer therapy development. Neutralizing TGF-β leads to increased production of neutrophil chemokines, which in turn recruit and activate neutrophil antitumor subsets. Table 1 shows some clinical trials based on neutrophil-associated tumor therapies against this backdrop. Directly targeting neutrophil receptors with drugs and therapies is also a hot topic in this area. For example, it has been shown that neutrophils are involved in inflammatory-induced lung cancer progression through the IL-8/CXCR2 pathway and NE and can be inhibited by selective CXCR2 inhibition. CXCR1 and CXCR2, chemokine receptors expressed on neutrophils and g-MDSCs, are promising targets for preventing breast and lung cancer metastasis by targeting neutrophils with CXCR1/2 agonists.
Table 1. Clinical trials after Phase II based on neutrophil associated tumor therapies (Modeled after lee et al)
| Drug | Cancer Applications | Phase | Clinicaltrails.gov No |
| Reparixin | Reparixin | II | NCT02370238 |
| Galunisertib | Glioblastoma | II | NCT01582269 |
| Napabucasin | Colorectal Cancer | III | NCT02753127 |
| Tigatuzumab | Breast Cancer | II | NCT01307891 |
Current clinical reports on neutrophil-related tumor immunotherapy include a 78-year-old man with squamous lung cancer and brain metastasis who was treated with granulocyte colony-stimulating factor (G-CSF) after other therapies failed to improve his condition. Unfortunately, the patient eventually died due to neurological problems.
Despite the potential of neutrophil-based immunotherapy, its progress has not been smooth in the clinic due to heterogeneity. The heterogeneity of the immune microenvironment is considered one of the important reasons for drug resistance, recurrence, and poor prognosis of tumors. However, in recent years, immunotherapy and related combination therapy programs have brought hope to patients with advanced tumors.
A recent research paper published in Nature has defined five immune microenvironment subtypes of liver cancer with single-cell precision, named the TIMELASER typing system. It is the first study to fully reveal tumor-associated neutrophil heterogeneity. In this study, the researchers performed scRNA-seq analysis of 160 samples of 124 treatment-naive patients, covering all cell populations across PLC (Primary liver cancer), including HCC, ICC, and CHC. They identified a total of 89 TIME (Tumor immune microenvironment) cell clusters among 1,092,172 cells obtained.
Further analysis found the enrichment of multiple neutrophil subsets in TIME-ISM (immune suppressive myeloid). These neutrophil subsets were associated with poor prognosis, and their scarcity led to further examination of neutrophils. The existence of neutrophils in PLC was confirmed, and it was observed that ICC has significantly more neutrophils than HCC, as shown using mIHC (multicolour immunohistochemistry).
Through hierarchical cluster analysis, the researchers identified five different immune microenvironment subtypes, including immune activation type, myeloid enrichment immunosuppression type, matrix enrichment immunosuppression type, immune rejection type, and immune retention type. Interestingly, two neutrophil subsets, CCL4+ TAN and PD-L1+ TAN, were found to promote tumor growth through different mechanisms. The former promotes tumor growth by recruiting tumor-related macrophages, while the latter inhibits the killing function of CD8+ T cells. A series of experiments have verified these findings.
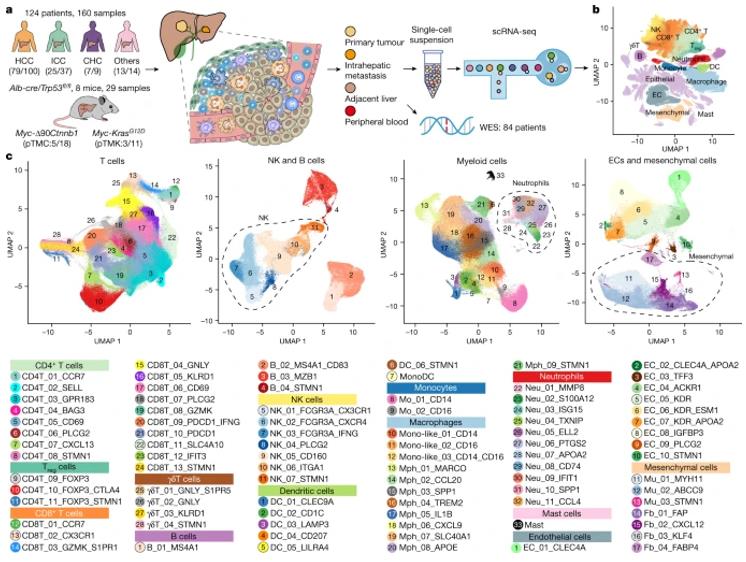 Figure 3. The single-cell landscape of 124 patients with liver cancer.
Figure 3. The single-cell landscape of 124 patients with liver cancer.
Research on neutrophil-based tumor immunotherapy is advancing rapidly, with crucial functions of neutrophils being discovered in liver repair by promoting the conversion of pro-inflammatory Ly6ChiCX3CR1lo monocytes/macrophages to pro-resolving Ly6CloCX3CR1hi macrophages. Researchers have found that adoptive transfer of WT neutrophils, rather than Nox2−/− (genetic deficiency of NADPH oxidase 2) neutrophils, rescues impaired phenotypic conversion of macrophages in neutrophil-depleted mice, and the important functions of neutrophils and macrophages in response to oxidative liver injury were described during the experiment. These findings provide the prospect of the application of neutrophil transfer in clinical treatment.
In addition, monoclonal antibodies targeting neutrophils provides new avenues for the treatment of various inflammatory and autoimmune conditions. By selectively targeting these cells, monoclonal antibodies can effectively modulate the body's immune system, reducing unwanted inflammation and tissue damage. This targeted approach not only promises enhanced efficacy in treating diseases characterized by excessive neutrophil activity but also minimizes potential side effects associated with broader immunosuppressive therapies.
While a growing number of studies on neutrophil-based immunotherapy have been conducted in mice, the reliability of the data from humans is uncertain due to the uneven nature of tumors in human patients compared to mouse models. More exploration is required to identify biomarkers or cellular and molecular pathways that regulate neutrophil phenotype and function in human cancer patients. Despite this, the broad potential of neutrophils in immunotherapy is promising and worth further investigation.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION