Neutrophils, or polymorphonuclear leukocytes, represent a vital subset of white blood cells integral to the innate immune response. They constitute the predominant leukocyte population in the bloodstream and fulfill essential functions including phagocytosis, release of microbicidal substances, formation of neutrophil extracellular traps (NETs), and secretion of cytokines and chemokines. While neutrophils are pivotal for combating infections, aberrant or excessive neutrophil activation can lead to tissue damage and inflammation, contributing to the pathogenesis of conditions such as sepsis, autoimmune disorders, and chronic inflammatory diseases.
As such, monoclonal antibodies targeting neutrophils have emerged as a focal point in therapeutic investigations, particularly in inflammatory and autoimmune disorders characterized by neutrophil-driven inflammation. These antibodies offer a targeted approach to modulate neutrophil function and dampen inflammatory responses, aiming to mitigate tissue damage and restore immune homeostasis in disease settings. Ongoing research endeavors continue to explore the therapeutic potential of neutrophil-targeted monoclonal antibodies across various clinical contexts.
CD11/CD18, also known as the leukocyte integrins or β2 integrins, are a family of cell surface adhesion molecules primarily expressed on neutrophils. These integrins play crucial roles in neutrophil adhesion and migration, particularly during inflammatory responses and immune surveillance. The CD11/CD18 integrins mediate neutrophil adhesion to endothelial cells by binding to endothelial cell adhesion molecules (e.g., ICAM-1) expressed on inflamed blood vessels. This interaction facilitates the firm adhesion of neutrophils to the endothelium. Subsequently, CD11/CD18 integrins promote neutrophil migration across the endothelial barrier (diapedesis) into the tissue, a critical step in the inflammatory response.
Dysregulated CD11/CD18 integrin signaling is implicated in various inflammatory and autoimmune diseases, including inflammatory bowel disease (IBD), rheumatoid arthritis, and vasculitis. Abnormalities in CD11/CD18 integrin function contribute to excessive neutrophil infiltration and tissue damage in these pathological conditions.
Monoclonal antibodies targeting CD11/CD18 integrins can inhibit neutrophil adhesion and migration, thereby reducing inflammation and attenuating immune-mediated diseases. Examples of such antibodies include natalizumab and efalizumab, which interfere with integrin-mediated neutrophil functions. CD11/CD18 integrins are critical regulators of neutrophil adhesion and migration during inflammation. Targeting these integrins with monoclonal antibodies represents a promising therapeutic strategy for modulating immune responses and mitigating inflammatory diseases characterized by dysregulated neutrophil activation and tissue damage. Further research is needed to optimize the clinical utility and safety of CD11/CD18-targeting monoclonal antibodies in inflammatory disorders.
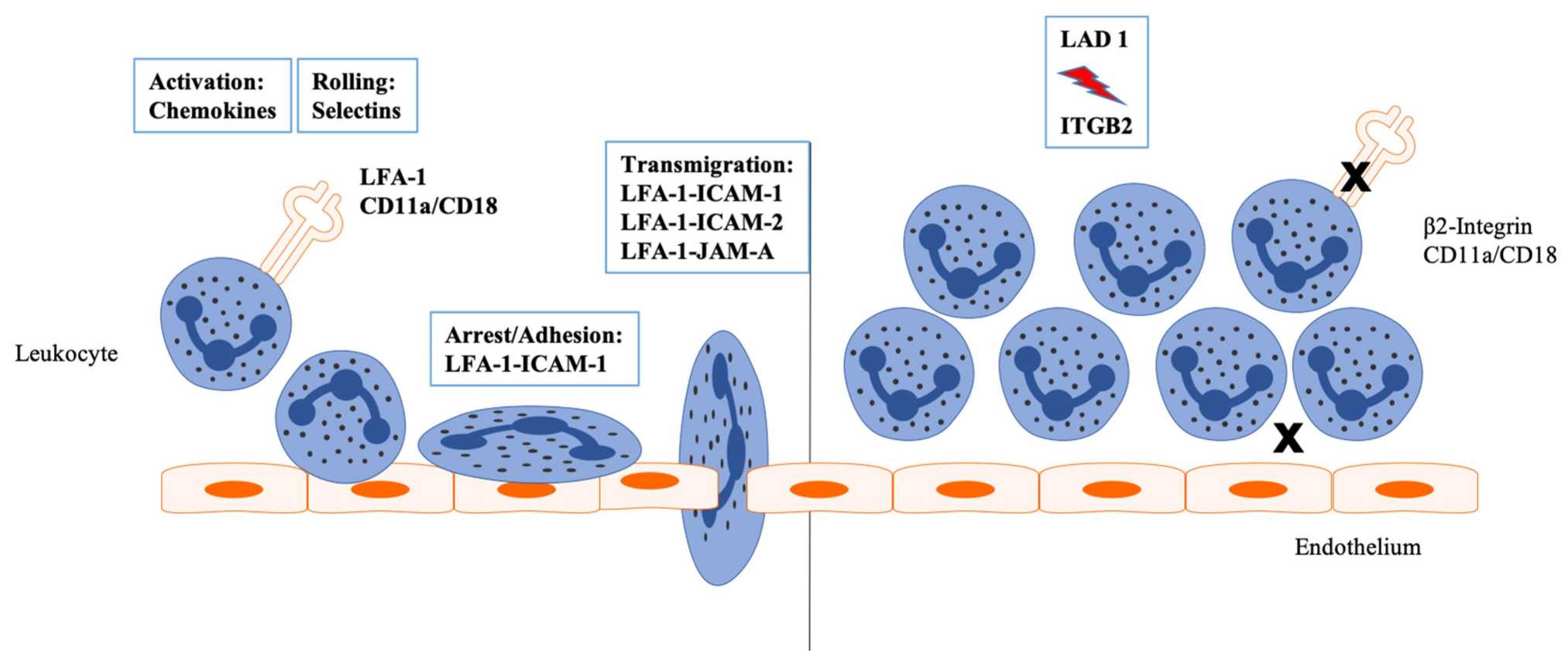 Fig 1. Inflammation leads to an activation of the endothelium by endogenous and exogenous stimuli. (Julia, 2022)
Fig 1. Inflammation leads to an activation of the endothelium by endogenous and exogenous stimuli. (Julia, 2022)
Interleukin-17A (IL-17A) is a pro-inflammatory cytokine primarily derived from T helper 17 (Th17) cells, a subset of CD4+ T lymphocytes, as well as other immune cells including neutrophils, mast cells, and innate lymphoid cells. It plays a pivotal role in orchestrating immune responses, particularly by promoting inflammation, neutrophil recruitment and activation, and maintenance of epithelial barrier integrity. IL-17A functions through binding to its receptor IL-17RA, triggering downstream signaling pathways that culminate in the production of pro-inflammatory cytokines, chemokines, and antimicrobial peptides.
Dysregulated IL-17A signaling is implicated in the pathogenesis of various autoimmune and inflammatory diseases, encompassing psoriasis, rheumatoid arthritis, inflammatory bowel disease (IBD), and asthma. Therapeutically, efforts have focused on targeting IL-17A or its receptor as a strategy to mitigate inflammatory disorders.
Monoclonal antibodies directed against IL-17A, such as Secukinumab and Ixekizumab, have been developed to inhibit IL-17A-mediated neutrophil-driven inflammation. These antibodies are clinically utilized in the management of autoimmune conditions such as psoriasis and psoriatic arthritis, demonstrating efficacy in suppressing inflammation and ameliorating disease manifestations. Ongoing research continues to explore the therapeutic potential of IL-17A-targeted therapies across a spectrum of immune-mediated disorders.
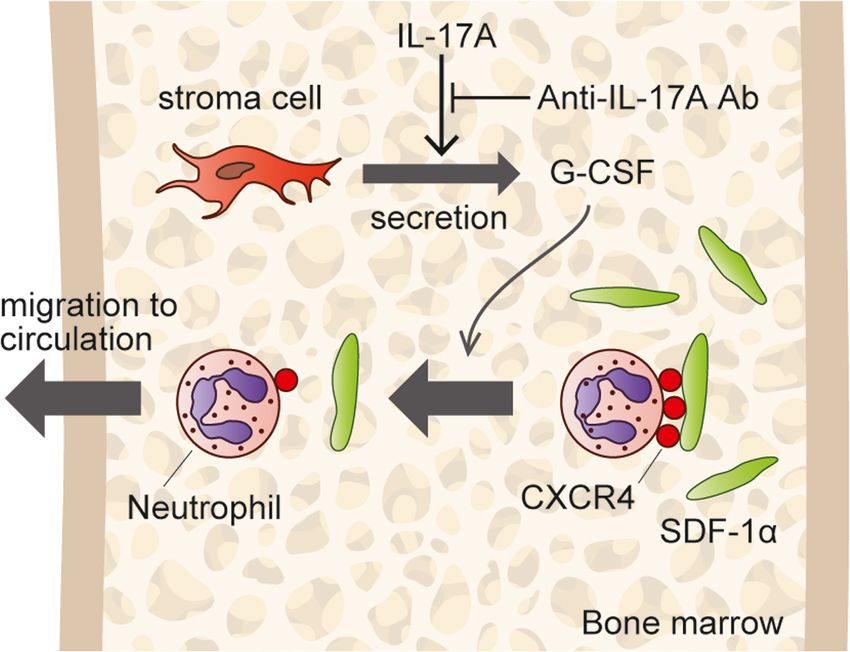 Fig 2. IL-17A stimulates bone marrow (BM) stroma cells to secrete G-CSF, and G-CSF mediates neutrophils. (Hiroshi, 2019)
Fig 2. IL-17A stimulates bone marrow (BM) stroma cells to secrete G-CSF, and G-CSF mediates neutrophils. (Hiroshi, 2019)
Interleukin-23 (IL-23) is a cytokine predominantly secreted by activated dendritic cells, macrophages, and other antigen-presenting cells, exerting a pivotal role in immune regulation, particularly in the context of neutrophil activation and autoimmune pathogenesis.
IL-23 acts on various immune cells, notably T cells, to stimulate inflammation and immune responses by binding to its receptor IL-23R. This interaction triggers downstream signaling pathways that induce the production of pro-inflammatory cytokines.
Dysregulated IL-23 signaling is implicated in the pathogenesis of autoimmune diseases such as psoriasis, inflammatory bowel disease (IBD), and ankylosing spondylitis, where elevated IL-23 levels in affected tissues correlate with disease severity. IL-23 serves as a critical driver of inflammation and immune dysregulation in autoimmune conditions, rendering it a promising target for therapeutic interventions employing monoclonal antibodies and other targeted therapies.
Monoclonal antibodies directed against IL-23, such as ustekinumab, exert their therapeutic effects by modulating neutrophil-mediated inflammation and are employed in the treatment of autoimmune diseases such as psoriasis and Crohn's disease. Ongoing research continues to explore IL-23-targeted therapies to optimize treatment outcomes in immune-mediated disorders.
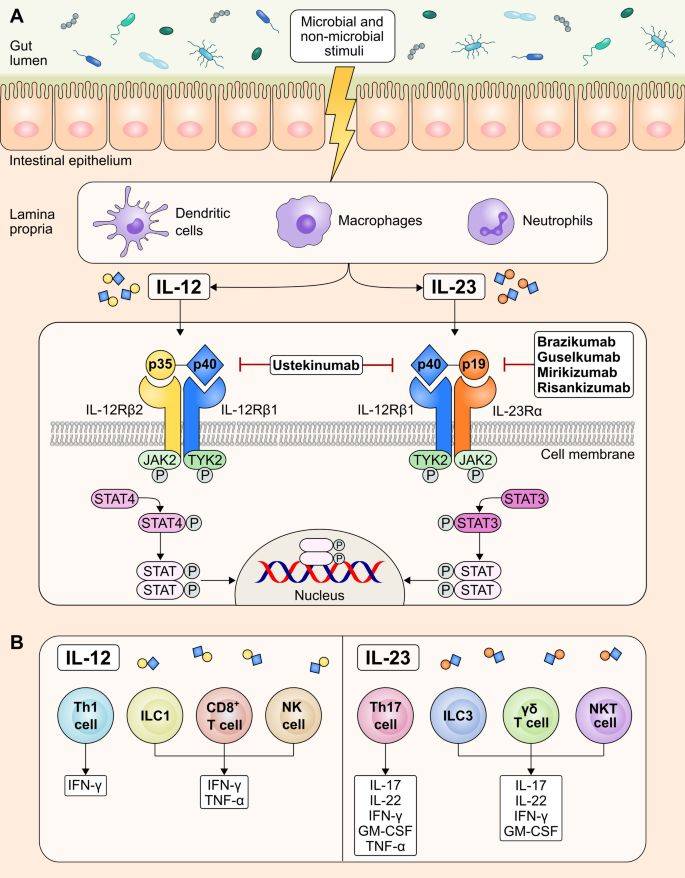 Fig 3. Targeting IL-23 for IBD (Sudheer, 2023)
Fig 3. Targeting IL-23 for IBD (Sudheer, 2023)
CXCR2 (C-X-C chemokine receptor type 2) is a G-protein coupled receptor predominantly expressed on neutrophils and other immune cells. It plays a pivotal role in orchestrating the migration (chemotaxis) of neutrophils towards sites of inflammation in response to chemokine signals. Chemokines such as IL-8 bind to CXCR2 on neutrophils, initiating intracellular signaling cascades that lead to cytoskeletal rearrangement and directed migration towards the chemokine source. However, dysregulated CXCR2 signaling is implicated in various inflammatory diseases, including chronic obstructive pulmonary disease (COPD), asthma, rheumatoid arthritis, and inflammatory bowel disease (IBD). Excessive neutrophil recruitment and activation via CXCR2 contribute to tissue damage and inflammation in these pathological conditions.
Monoclonal antibodies and small molecule inhibitors that block CXCR2 signaling aim to mitigate excessive neutrophil infiltration and ameliorate inflammation associated with inflammatory diseases. Therapeutic targeting of CXCR2 represents a promising strategy to modulate neutrophil-mediated inflammation and manage conditions characterized by aberrant neutrophil infiltration and tissue damage. This approach holds potential for developing novel interventions to mitigate inflammatory responses and improve clinical outcomes in patients with inflammatory disorders.
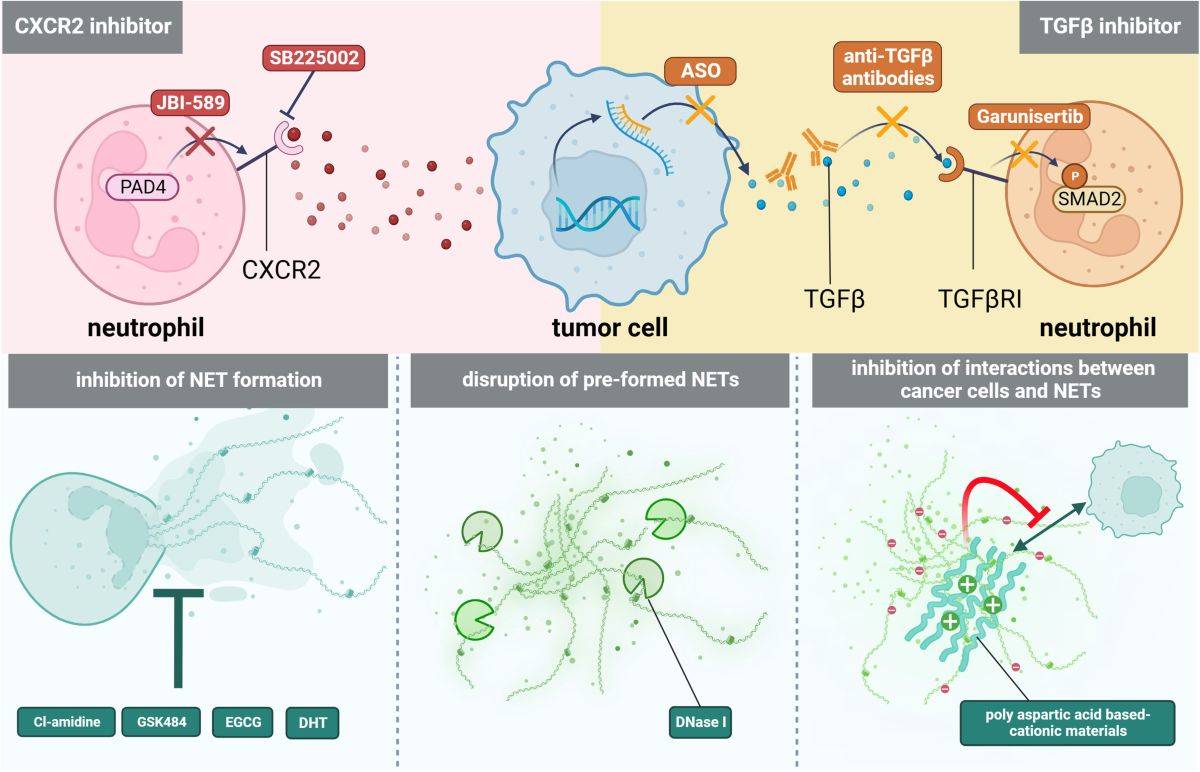 Fig 4. Tumor immunotherapy targeting CCR2 (Xinyu, 2024)
Fig 4. Tumor immunotherapy targeting CCR2 (Xinyu, 2024)
Presently, an expanding comprehension of neutrophil biology has illuminated and encouraged the development of novel treatment pathways. In order to cater to the demands of our global customers and streamline the transition of dendritic cell research into clinical applications, Creative Biolabs enthusiastically offers a diverse range of readily used neutrophil products that have undergone rigorous quality assurance measures. Please don't hesitate to contact us and inquire about a suitable product.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION